Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 1 - Jan. /Feb. of 2008
Vol. 41 nº 1 - Jan. /Feb. of 2008
|
CASE REPORT
|
|
Agenesis of the internal carotid artery: a case report |
|
|
Autho(rs): William da Silva Neves, Milton Yochiharu Kakudate, Crescêncio Pereira Cêntola, Raphael Gouveia Garzon, Américo Poça d'Água, Rafaelo Sanches |
|
|
Keywords: Internal carotid artery, Agenesis, Syncope |
|
|
Abstract:
IMD, formerly Resident in Radiology at Instituto de Radiodiagnóstico Rio Preto (Ultra-X), São José do Rio Preto, SP, Brazil
INTRODUCTION The embryonal development of the cerebral arterial circulation occurs in seven phases. Certain alterations in this process may lead to agenesis or hypoplasia of the carotid vessels. According to Streeter(1), Padget(2), and McLone & Naidich(3) two branches of the primitive internal carotid artery (ICA) develop early in the embryogenesis, originating from the third aortic arch. In its primitive form, the ICS reaches the cephalic region up to the level of the Rathke's pouch where two primary divisions will occur. Only one cranial branch will extend anteriorly to supply the developing forebrain. In summary, the anterior choroidal, middle cerebral, anterior cerebral and primitive olfactory arteries will develop from this vessel. Posteriorly, another branch will give rise to the posterior choroidal, diencephalic and mesencephalic arteries. As these branches advance caudally, anastomosis will be made with the developing longitudinal neural arteries supplied by the trigeminal artery connections to the primitive ICA. Ultimately, this pattern describes the first and second developmental phases and results in the adult cerebral circulation model to be established after the alterations along the other five phases as follows: the third phase, involving the vertebral arteries; the fourth phase, involving the anterior cerebral artery; the fifth phase, ophthalmic arteries; the sixth phase, the circle of Willis; and the seventh or fetal phase(4).
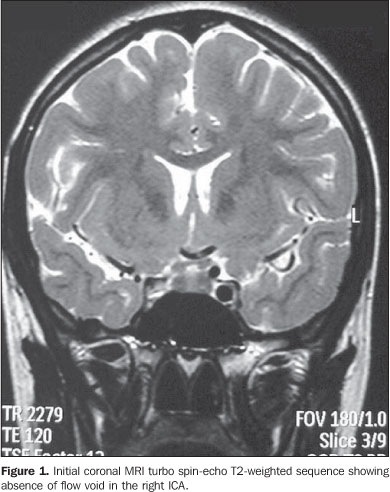
CASE REPORT A female, caucasian, 14-year-old patient, coming from the interior of the state of São Paulo, who, 15 days ago, had undergone an episode of syncope witnessed by her relatives, where transitory signs of somnolence and dysarthria were observed. The patient was immediately referred to a neurologist. Both during the clinical examination and magnetic resonance imaging (MRI) scanning, the patient presented asymptomatic, with no new episode of syncope or other complaints being reported. The suspicion of ICA agenesis was raised by MRI in the absence of the typical flow void in the ICA region, on the spin-echo sequences (Figure 1).
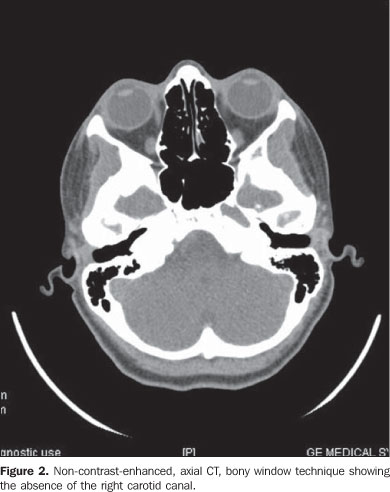
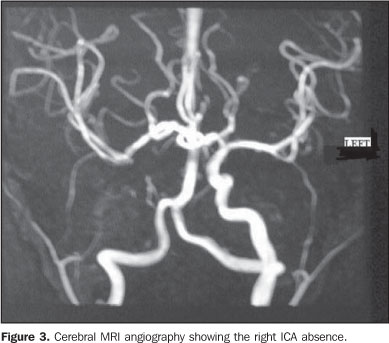
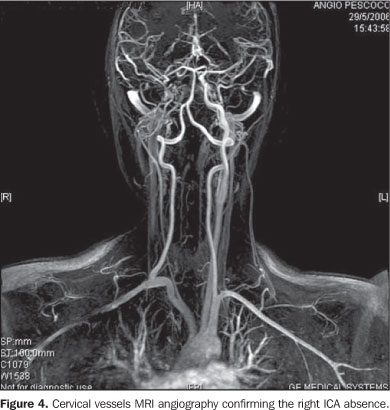
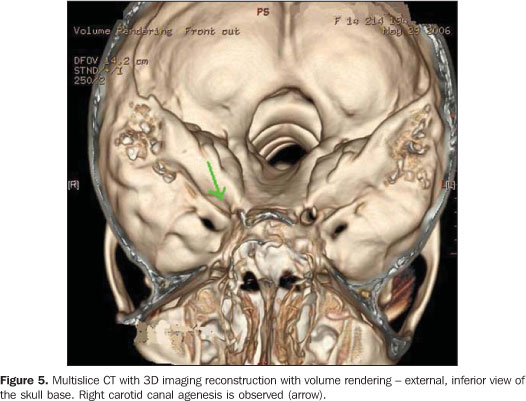
Considering the suspicion of agenesis or hypoplasia of the right ICA, the patient was submitted to non-contrast enhanced computed tomography (CT) and MRI angiography of the brain and cervical vessels, for diagnostic confirmation. The CT study was aimed at differentiating a congenital from an acquired agenesis of the bony carotid canal, but the absence of the right carotid canal was observed. The MRI angiography of the brain and cervical vessels was performed in an attempt to depict a global picture of the cerebral arterial network, and the absence of the right ICA from the aortic arch (Figures 2, 3 and 4).
Additionally, the patient was submitted to multislice CT, in an attempt to demonstrate some other skull bone abnormality, but only a confirmation of the other studies findings was observed by means of the 3D imaging reconstruction with volume rendering, demonstrating the absence of the right carotid canal.
DISCUSSION An extremely rare incidence of ICA agenesis has been reported, with few scarce tens of cases being worldwide reported, maybe, to some extent, because the dysgenesis is generally asymptomatic in the greatest majority of patients. This occurs because there is a sufficient cerebral circulation supplied by anastomosis in the circle of Willis, intracavernous and external carotid artery anastomosis, besides persistent embryonal arteries. In these cases, the patients are referred for medical assistance because of complications resulting from abnormalities associated with carotid artery agenesis(5–7). The ICA agenesis is usually unilateral, although there are reports of asymptomatic bilateral agenesis(8). If the agenesis is detected by MRI angiography, it must be confirmed by CT, in an attempt to find hypoplasia or absence of the carotid canal. Generally, the main secondary source of blood supply is the vertebrobasilar system (in cases of bilateral agenesis) or the dominant ICA (in cases of unilateral agenesis or hypoplasia)(7). The main findings associated with these anomalies are transsphenoidal encephaloceles, circle of Willis aneurysms, and an extensive rete mirabilis in the cranial base. Intracranial aneurysms are found in about 25% of cases of symptomatic internal carotid artery agenesis with intracranial hemorrhagic manifestations(5,6,9,10). Also, other less frequent findings are reported. There are few cases where agenesis is associated with neuropsychomotor development delay, agenesis of the corpus callosum and persistent cavum vergae, in patients with bilateral agenesis. Few cases of unilateral agenesis in association with arachnoid cyst are reported(11,12). Also, there is a report about association of megadolichobasilar anomaly and olivopontocerebellar atrophy with unilateral ICA agenesis(13), besides rare cases of hypopituitarism associated with unilateral artery agenesis, although intracavernous anastomosis seem to be efficient(14,15). The absence of clinical or neurological symptoms of ischemia in the majority of patients with unilateral or bilateral agenesis or hypoplasia of the ICA leads to the assumption that, most of times, perfusional deficit is not present. However, 123-iodine singlephoton emission computed tomography (SPECT) can be utilized, and has already been utilized to demonstrate the absence of areas with perfusional deficit, considering that some few patients have experienced episodes of transitory ischemic accidents of unidenfied etiology(11). Likewise the present case, other cases of agenesis of the internal carotid artery have been incidentally found by MRI angiography. In these cases, it is always necessary to confirm if the hypoflow is caused by an acquired stenosis or dysgenesis of the vessel and the bony carotid canal. So, axial CT of the skull base should be performed to confirm the patency or dysgenesis of the carotid canal, corresponding to the vessel agenesis or hypoplasia(16). Multidetector CT with 3D reconstruction also is useful in cases of dubious diagnosis.
CONCLUSION In this brief case report, there is no intention to affirm that agenesis of the ICA should be always suspected. However, it may be concluded that a detailed and methodological evaluation of the carotid canals and ICA flow void in the investigation of primary or acquired stenosis (a usual finding in patients with neurological complaints) may lead to the finding of this anomaly that, although asymptomatic, may be associated with other potentially severe malformations and disorders. The carotid canals should always be evaluated during the reading of routine cranial CT studies with axial slices and bone window images, considering that this is the confirmatory sign of the ICA absence, and does not imply an increase neither in the acquisition time nor in the cost of the study. The finding of an episode of syncope in this patient may be associated with a transitory ischemic accident.
REFERENCES 1. Streeter GL. The developmental alterations in the vascular system of the brain of the human embryo. Philadelphia, PA: Lippincott Williams & Wilkins Contrib Embryol Carnegie Inst. 1918;8:5–38. [ ] 2. Padget DH. The development of the cranial venous system in man, from the viewpoint of comparative anatomy. Philadelphia: Lippincott Williams & Wilkins Contrib Embryol Carnegie Inst. 1957; 36:81–140. [ ] 3. McLone DG, Naidich T. Embryology of the cerebral vascular system. In: Edwards MSB, Hoffman HJ, editors. Cerebral vascular disease in children and adolescents. Baltimore, MD: Williams & Wilkins; 1989:1–16. [ ] 4. Truwit CL, Barkovich AJ. Disorders of brain development. In: Atlas SW, editor. MRI of the brain and spine. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997:1–25. [ ] 5. Blustajn J, Netchine I, Frédy D, et al. Dysgenesis of the internal carotid artery associated with transsphenoidal encephalocele: a neural crest syndrome? AJNR Am J Neuroradiol. 1999;20: 1154–7. [ ] 6. Quint DJ, Silbergleit R, Young WC. Absence of the carotid canals at skull base CT. Radiology. 1992;182:477–81. [ ] 7. Tasar M, Yetiser S, Tasar A, et al. Congenital absence or hypoplasia of the carotid artery: radioclinical issues. Am J Otolaryngol. 2004;25: 339–49. [ ] 8. Rumboldt Z, Castillo M, Solander S. Bilateral congenital absence of the internal carotid artery. Eur Radiol. 2003;13 Suppl 6:L130–2. [ ] 9. Gailloud P, Clatterbuck RE, Fasel JHD, et al. Segmental agenesis of the internal carotid artery distal to the posterior communicating artery leading to the definition of a new embryologic segment. AJNR Am J Neuroradiol. 2004;25:1189–93. [ ] 10. Cali RL, Berg R, Rama K. Bilateral internal carotid artery agenesis: a case study and review of the literature. Surgery. 1993;113:227–33. [ ] 11. Kidooka M, Okada T, Handa J. Agenesis of the internal carotid artery – report of a case combined with arachnoid cyst in a child. No To Shinkei. 1992;44:371–5. [ ] 12. Owada Y, Sakuta Y. Bilateral carotid artery agenesis with corpus callosum hypogenesis – a case report. No To Shinkei. 1995;47:589–94. [ ] 13. Okabe S, Oda N, Ishii M. Agenesis of left internal carotid artery associated with megadolichobasilar anomaly and olivopontocerebellar atrophy. Rinsho Shinkeigaku. 1989;29:1395–400. [ ] 14. Moon WJ, Porto L, Lanfermann H, et al. Agenesis of internal carotid artery associated with congenital anterior hypopituitarism. Neuroradiology. 2002;44:138–42. [ ] 15. Baysefer A, Akay KM, Tasar M, et al. Congenital absence of internal carotid artery associated with hypogonadotropic hypogonadism – a case report. Vasc Endovascular Surg. 2002;36:457–60. [ ] 16. Graham CB 3rd, Wippold FJ 2nd, Capps GW. Magnetic resonance imaging in internal carotid artery agenesis with computed tomography and angiographic correlation – case reports. Angiology. 1999;50:847–53. [ ]
Received September 14, 2006. Accepted after revision March 23, 2007.
* Study developed in the Centro Regional de Radiologia Intervencionista Vascular do Hospital Beneficência Portuguesa de São José do Rio Preto, and Instituto de Radiodiagnóstico Rio Preto (Ultra-X), São José do Rio Preto, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554