Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 1 - Jan. /Feb. of 2008
Vol. 41 nº 1 - Jan. /Feb. of 2008
|
NEWS IN RADIOLOGY
|
|
Acquisition and manipulation of computed tomography images of the maxillofacial region for biomedical prototyping |
|
|
Autho(rs): Maria Inês Meurer, Eduardo Meurer, Jorge Vicente Lopes da Silva, Ailton Santa Bárbara, Luiz Felipe Nobre, Marília Gerhardt de Oliveira, Daniela Nascimento Silva |
|
|
Keywords: Rapid prototyping, Biomedical prototyping, Computed tomography |
|
|
Abstract:
IPhD of Clinical Stomatology, Associate Professor, Department of Pathology, Universidade Federal de Santa Catarina (UFSC), Florianópolis, SC, Brazil
INTRODUCTION Accuracy in the diagnosis and treatment planning for patients represents a challenge to surgical teams, particularly in more complex cases such as facial deformities. Facial deformities are relatively frequent, requiring complex, time-consuming and expensive treatments. Exorbitant resources are invested in functional, aesthetic and social rehabilitation of patients with facial deformities(1,2), considering the necessity of long hospitalization periods, social security costs during the recovery period, number of reconstructive surgical procedures, and costs of interdisciplinary surgical teams as well as clinical ancillary personnel(2,3). Besides congenital causes, labor-, traffic and violence-related accidents show up as acquired causes of facial deformities; in Brazil, the number of hospital admissions as a result of accidents and violence is alarming(4). Additionally, patients with severely affected by facial deformities present with lower level of social integration than patients with alteration in limbs or other parts of their body. Computed tomography (CT) is the method of choice among the diagnostic imaging techniques utilized for preoperative evaluation of these patients. Besides the essential analysis of 2D images, 3D visualization allows a privileged access to the structures of interest, particularly for the surgeon, highlighting some aspects which otherwise only could be accessed by means of a "mental reconstruction" of tomographic images. Despite the unquestionably elucidative character of virtual 3D images, some distance still persists between the virtual model and the actual handling of anatomic structures during a surgical procedure. Biomedical prototyping from CT images has shown to be an innovative solution to minimize this distance. Similarly to products manufacturing, where prototypes are utilized to improve the final quality, allowing the detection of possible mistakes at early stages of the product development(5), biomedical prototyping allows the construction of physical objects reproducing anatomic structures and only is possible by means of an integration of medical images acquisition and manipulation with computer-aided design (CAD) and rapid prototyping (RP) techniques, therefore involving multidisciplinary teams. With the direct visualization and manipulation of an anatomic replica of bone structures, biomedical prototyping allows, for example, an accurate measurement of such structures as well as simulation of osteotomies and resection techniques. As a result, a decrease in the surgical time, anesthesia period and infection risk can be observed, with a decrease in costs of the global treatment and better clinical results(1,6,7). Additionally, the utilization of these prototypes improves the communication between practitioners and patients, aiding in the explanation about the surgical procedure and in the obtention of the term of free and informed consent, besides being useful in the production of customized prosthetic implants(8–11). Biomedical prototypes also can be utilized for didactic purposes(9). The biomedical prototyping process requires an intense interaction of biomedical sciences, information technology and engineering. For the understanding of the process, and also the communication among the professionals involved it is necessary for engineers to learn concepts of diagnostic imaging and surgery, and for radiologists and surgeons to explore the world of information technology and manufacturing processes. This study is aimed at presenting a review of the key issues involved in the development of biomedical prototypes. The considerations presented in this paper result from the integration of a team including surgeons involved in the Program of Post-Graduation in Oralmaxillofacial Surgery and Traumatology of Pontifícia Universidade Católica do Rio Grande do Sul (CTBMF-PUCRS), engineers of the Division for Products Development at Centro de Pesquisa Renato Archer (CenPRA) and medical and dental radiologists of Universidade Federal de Santa Catarina (UFSC).
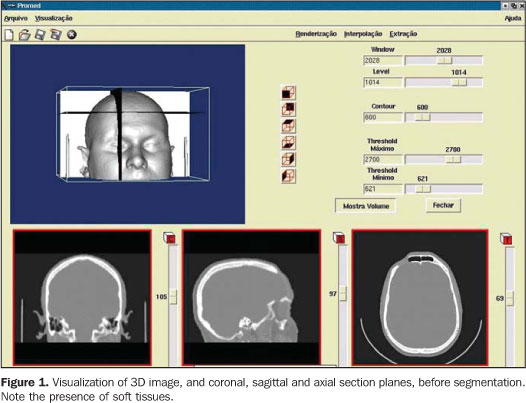
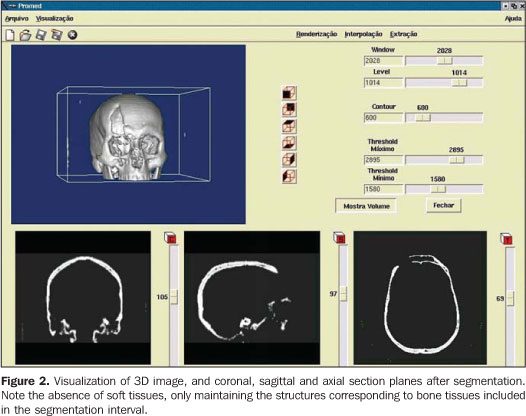
STEPS FOR THE CONSTRUCTION OF BIOMEDICAL PROTOTYPES 1. Patient selection The option for biomedical prototyping instead of less costly techniques, should be reserved for the cases where there is a real benefit to the patient, disregarding short-lived techniques and market appeals(6,7,12). This technique will be mostly useful in surgical procedures for which reliable techniques are not available or in cases where these techniques require further refinements and/or improvements. With the aid of a prototype during the surgical planning phase, a surgeon can elaborate the technique, evaluate details, optimize the procedure, anticipate difficulties and, mainly, the solution for these difficulties. Experiments involving the utilization of biomedical prototyping at CTBMF-PUCRS have demonstrated that the difficulties observed during the simulation of the surgical procedure with the biomodel are very similar to those found during the actual surgery, which corroborates the validity of these prototypes during the surgical planning. 2. Images acquisition It is desirable that a single volume of the whole segment to be studied is imaged utilizing thin sections. It is essential to take into consideration that the patient exposure to radiation is a limiting factor, and the radiologist is responsible for choosing the best images acquisition protocol, in order to achieve a balance between the prototype quality and radiation dose. Some steps are necessary to optimize the acquisition of the images for subsequent computer processing: – Theoretically, the slices thickness should be as low as possible to allow an appropriate image 3D reconstruction (excellent results can be achieved with 1 mm-thick slices). However, some times this is not feasible in cases of extensive areas to be scanned. – In cases where the area of interest includes only the face, the change in the acquisition plane from axial to coronal may reduce substantially the number of sections. – In the helical mode, an increase in the pitch may allow the obtention of more extensive volumes with thick slices, especially in multidetector CT equipment. This is a better solution than increasing the slice thickness. If the increase in the slices thickness is inevitable, the reconstruction interval for volume formation should be as small as possible. – The field of view (FOV) should cover the whole region of interest. For the face and skull, a FOV of approximately 250 mm is sufficient; even smaller FOVs can be utilized, depending on the area to be reproduced in the prototype. The smaller the FOV, the higher the image quality, because the available matrix is applied to a smaller area, reducing the pixel size and, consequently, the partial volume effect(13). – In principle, the gantry should not be inclined during the images acquisition, considering that some softwares for images processing do not allow the compensation of this inclination yet, producing prototypes with dimensional alterations(14). – The utilization of filters during images acquisition is controversial. Some studies have reported a higher rate of artifacts when bone filters are utilized during images acquisition(15,16). – Artifacts related to metal dental restorations should be subsequently removed by means of graphic computing tools, although this is a time-consuming and boring process which many times affects negatively the final outcomes. Aiming at minimizing the productions of such artifacts, the patient should be positioned in the occlusal plane (occlusal surfaces of the teeth) parallel to the axial plane. With this strategy, artifacts are restricted to the region of the dental cusps, reducing the number of sections to be manually edited. 3. Image files storage and transference The lacking standardization of image formats is one of the issues to be considered. Digital imaging and communications in medicine (DICOM)(17) is currently the globally accepted industry standard the allows higher interoperability between computer systems and medical equipment. Not all CT equipment (particularly the oldest ones) is able export DICOM files. So, it is important to previously confirm with the prototyping center the compatibility between the images format and the editing application software. The files may be stored in any media available, provided the data storage capacity of such media is high. Typically, CDs, DAT tapes and optical disks are utilized as recording media. Rewritable (R/W) CDs should be avoided since they may not be recognized by some editing application softwares(18), or even present a higher data volatility, difficulting the future records retrieval. The data volume may pose a problem for the image files transfer. Each image in the DICOM format with 512 × 512 pixels acquisition matrix generates a 512 Kbytes file/slice. A skull CT in compliance with the requirements for the development of an appropriate prototype may generate a data volume of approximately 100 Mbytes. So, compacting tools can be useful at the moment of data transfer. Broadband networks allow the data transfer via internet with the file transfer protocol (FTP) directly to the prototyping workstation. The files availability in local restricted access networks (allowing downloads by the prototyping workstation), or even the mailing of CDs represent additional alternatives for the images transference. Sending image files attached to emails may be an additional option, although the file size may frequently cause the file rejection by the server. As far as data access is concerned, security-related issues should be taken into consideration. 4. Images manipulation Images manipulation and editing is performed with the aid of specific application softwares, and this process requires a close interaction between biomedical specialties and engineering(19). The aim of this process is the images segmentation for separating data of interest from the information set provided by CT. In the case of the prototyping for oralmaxillofacial surgery where the study object is a bone specimen, the images segmentation is aimed at separating the bony portion from the adjacent tissues (Figures 1 and 2).
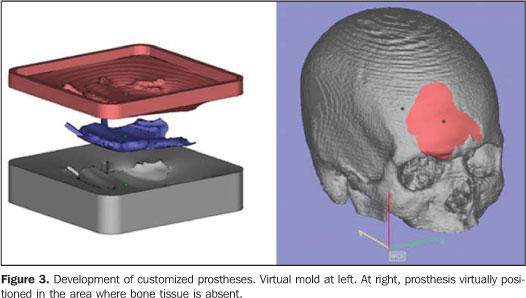
Among the tools available for images segmentation, the threshold is fairly utilized, and is based on the definition of density intervals expressing, for example, only the voxels corresponding to bone tissues. Should this interval be erroneously determined, the so called dumb-bell effect will occur, with a possible structures suppression or alteration during the process(15). In some cases, the manual images editing is required, with tools such as cut, erase and select. This editing is particularly useful in case of imaging artifacts originating from dental prostheses or restorations. CenPRA, by means of the project Prototipagem Rápida na Medicina (Promed) (Rapid Prototyping in Medicine), has developed the InVesalius free software, a pioneering application for medical images processing aimed at the production of biomedical prototypes. The first version of this software is already available to practitioners and health institutions in the area of biomedicine (free licenses may be requested at: http://www.cenpra.gov.br/promed). InVesalius, includes some algorithms offering3D visualization, segmentation, and 2D/3D reformatting resources. Additionally, the software offers a conversion process function, allowing images conversion into a format recognized by RP equipment. The integration between CAD and medical systems facilitates the manipulation and modeling of objects, allowing virtual images of segmented structures to be manipulated as if they were pieces of a puzzle or biomechanic prototype. Additionally, CAD systems are appropriate for defining mirroring procedures utilizing the contralateral symmetry of the face, allowing the planning and development of customized prosthesis, including by simulating the prosthesis assembly on a 3D model (Figure 3). The construction of the customized prosthesis can be done by modeling the structure which will replace the injured area, or by means of CAD systems operations, generating a 3D model of a mold; then, the mold obtained by RP is utilized to give a shape to the material to be implanted (typically implantable polymers or ceramic materials). It is important that typical contractions of some of these materials are taken into consideration in the molds construction. For implantable metallic materials, the mold may be utilized for generating wax models to be utilized, for example, in the microfusion process.
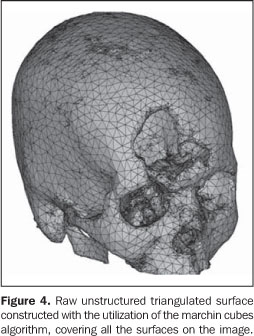
5. Images conversion The CT images cannot be directly processed by prototyping devices for two major reasons: firstly, the format of the file generated by the tomograph is not supported by the prototyping devices; secondly, the tomographic slices thickness usually ranges between 1 mm and 5 mm, and is considerably higher than those utilized in RP, about 0.1 mm. Additionally, RP processes utilize data originated from CAD-3D systems modeled by surfaces or solids, while tomographic images are represented by voxels. So, 2D tomographic images must be three-dimensionally reformatted and converted into a image format utilized in the RP processes; STL is the standard format, the model being represented by a raw unstructured triangulated surface(20,21) (Figure 4). Then, the STL file is processed by a specific prototyping software for correction of eventual inconsistencies in the surface geometry, triangles vertexes, optimization of the triangles number, as well as in the selection of the construction orientation most appropriate to the piece geometry — a phase known as process planning. Following this phase, the virtual STL model is resliced in parallel layers to allow the prototype construction.
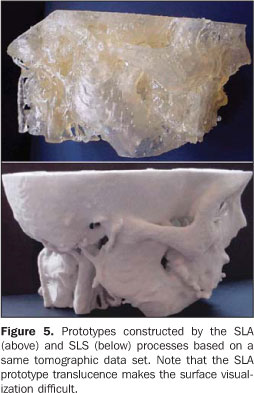
Hundreds to thousands of triangle facets constitute the model of a complex structure like the human skull, generating excessively-sized files and difficulting the images processing. 6. Prototype construction For the prototype construction, the STL files must be sent to the rapid prototyping workstation (usually, the computer where the images are processed is not located at the same place of the prototyping workstation). The files transfer can be accomplished by a media appropriate for data transmission (CD, e-mail, FTP, local network). Once the transfer is completed, the model construction is automatic in the majority of processes. The construction may take several hours to be completed, depending on the number of layers and the prototype height. Table 1 shows an overview of the advantages and disadvantages of the main rapid prototyping processes utilized in Brazil(20) for biomedical applications. This table is not aimed at undervaluing or otherwise valuing equipment of manufacturers, but rather to provide concise information to aid health professionals in their decision making about the process to be adopted according to their investment requirements and conditions. It is important to take into consideration that the costs for equipment acquisition and operation is high requiring specialized personnel, and not always the return on investment is rewarding. For this reason, the greatest majority of users opt for acquiring the services rather than the equipment. Figure 5 illustrates biomedical prototypes produced by the processes SLA and SLS and based on a same data set. Some clinical cases may be found at http://www.cenpra.gov.br/promed/casos.htm.
FINAL CONSIDERATIONS Biomedical prototypes present a high potential in the selection of new, frequently alternative, therapeutic approaches. In Brazil, the utilization of such prototypes is still restricted, particularly because of the high production costs involved and poor equipment availability in the country. Besides the high cost, the time-consuming nature of the process (from the images acquisition up to the prototype production) complicates the utilization of this method in routine surgical procedures, even if there is an indication. However, it is probable that such limitation will be overcome either by the technological development, or by the interdisciplinary utilization of this method, increasing the accessibility to biomedical prototypes. As far as future prospects are concerned, it is important to highlight the relevance of this technology in the tissue engineering. This is also a multidisciplinary domain of the science that combines and apply engineering principles and life sciences, in an attempt to develop biological substitutes to restore, maintain and improve functions of a determined tissue or organ(22). Studies can already be found in the literature, demonstrating that tissues can really be reproduced using this technique(23,24). The production of a model by rapid prototyping, in a specific shape and with a structure appropriate for adherence and cellular reproduction could be a feasible solution for reconstructive treatments in humans(25). An even more spectacular advance is the possibility of directly producing organs by means of rapid prototyping-like mechanisms called bioprinting(26). The experience gained by the authors with the obtention of biomodels has allowed the researchers to exchange knowledge essential for the development of each specific area. CenPRA has experienced the opportunity to have its software utilized by a group of specialists who ultimately constitute its target public. Differently from the majority of specialized softwares, the InVesalius was designed for running on personal computers, processing medical images and allowing their dynamic interpretation according to the diagnostic requirements or even during surgical procedures. More than 1500 free licenses have already been granted to specialists of different areas of knowledge in Brazil and several other countries. Additionally, CenPRA , in association with partners in the biomedical area, has been developing methodologies for surgical planning and biomodels construction. Biomedical prototypes and the InVesalius software have been utilized in the Program of Post-Graduation in Oralmaxillofacial Surgery and Traumatology at PUCRS in the resolution of several surgical ceases. Besides the treatment of facial deformities, these prototypes have been utilized in implantodontics and in the treatment of patients with temporomandibular joint ankylosis, giving both the Institution's patients and graduating professionals the opportunity to access the recent technologies. Additionally PUCRS, in association with CenPRA, has developed researches on the accuracy of rapid prototyping biomodels for surgical planning purposes(16) and prototypes construction based on magnetic resonance imaging. At UFSC, radiologists have been studying the relation between CT images acquisition parameters and prototypes quality, aiming at a less empirical CT images acquisition and processing for biomedical prototypes construction. The main objective of this undertaking as a whole is to utilize technological resources and applied research to offer better treatments, respecting the dignity of patients who could achieve a better functional and aesthetic rehabilitation with these methods.
REFERENCES 1. Peckitt NS. Stereoscopic lithography: customized titanium implants in orofacial reconstruction. Br J Oral Maxillofac Surg. 1999;37:353–69. [ ] 2. Sanghera B, Naique S, Papaharilaou Y, et al. Preliminary study of rapid prototype medical models. Rapid Prototyping Journal. 2001;7:275–84. [ ] 3. ATLS – Advanced Trauma Life Suport. Student manual. 6th ed. Chicago: American College of Surgeons; 1997. [ ] 4. Ministério da Saúde do Brasil. Morbidade hospitalar do SUS por causas externas – por local de internação – Brasil. [Acessado em: 18/5/2007]. Disponível em: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sih/cnv/eiuf.def [ ] 5. Silva JVL, Yamanaka MC, Saura CE. Rapid prototyping: concepts, applications, and potential utilization in Brazil. In: 15th International Conference in CAD/CAM Robotics and Factories of the Future; 1999; Águas de Lindóia, SP. [ ] 6. Kermer C, Lindner A, Friede I, et al. Preoperative stereolithographic model planning for primary reconstruction in craniomaxillofacial trauma surgery. J Craniomaxillofac Surg. 1998;26:136–9. [ ] 7. Meurer E. As tecnologias CAD-CAM em cirurgia e traumatologia bucomaxilofacial. (Tese de Doutorado). Porto Alegre: Pontifícia Universidade Católica do Rio Grande do Sul; 2002. Disponível em: http://www.cenpra.gov.br/promed/PDF/Tese_Meurer.pdf [ ] 8. D'Urso PS, Atkinson RL, Lanigan MW, et al. Stereolithographic (SL) biomodelling in craniofacial surgery. Br J Plast Surg. 1998;51:522–30. [ ] 9. James WJ, Slabbekoorn MA, Edgin WA, et al. Correction of congenital malar hypoplasia using stereolithography for presurgical planning. J Oral Maxillofac Surg. 1998;56:512–7. [ ] 10. Haex JKT, Poukens JMN. Preoperative planning with the use of stereolithographic model. Phidias Newsletter. 1999(3). [Acessado em: 22/3/2007]. Disponível em: http://193.74.1000.113/Medical/files/ph3.pdf [ ] 11. Petzold R, Zeilhofer HF, Kalender WA. Rapid protyping technology in medicine – basics and applications. Comput Med Imaging Graph. 1999; 23:277–84. [ ] 12. Perry M, Banks P, Richards R, et al. The use of computer-generated three-dimensional models in orbital reconstruction. Br J Oral Maxillofac Surg. 1998;36:275–84. [ ] 13. Cáceres KPS. Efeitos da variação da espessura do corte tomográfico e da largura do campo de visão (FOV) na reprodução de estruturas ósseas finas com a finalidade de prototipagem rápida – estudo in vitro. (Monografia). Florianópolis: Universidade Federal de Santa Catarina; 2005. [ ] 14. Kragskov J, Sindet-Pedersen S, Gyldensted C, et al. A comparison of three-dimensional computed tomography scans and stereolithographic models for evaluation of craniofacial anomalies. J Oral Maxillofac Surg. 1996;54:402–11. [ ] 15. Choi JY, Choi JH, Kim NK, et al. Analysis of errors in medical rapid prototyping models. Int J Oral Maxillofac Surg. 2002;31:23–32. [ ] 16. Silva DN. Análise do erro dimensional dos biomodelos de sinterização seletiva a laser (SLS) e de impressão tridimensional (3DP), a partir de imagens de tomografia computadorizada, na reprodução da anatomia craniomaxilar: estudo in vitro. (Tese de Doutorado). Porto Alegre: Pontifícia Universidade Católica do Rio Grande do Sul; 2004. [ ] 17. DICOM Standards Committee. DICOM Home Page. [Acessado em: 22/3/2005]. Disponível em: http://medical.nema.org [ ] 18. Kernan BT, Wimsatt JA 3rd. Use of a stereolithography model for accurate, preoperative adaptation of a reconstruction plate. J Oral Maxillofac Surg. 2000;58:349–51. [ ] 19. Silva JVL, Meurer E, Zavaglia CAC, et al. Rapid prototyping applications in the treatment of craniomaxillofacial deformities – utilization of bioceramics. Key Engineering Materials. 2003; 254-256:687–90. [ ] 20. Volpato N. Prototipagem rápida: tecnologia e aplicações. São Paulo: Edgard Blücher; 2007. [ ] 21. Souza MA, Centeno TM, Pedrini H. Integrando reconstrução 3D de imagens tomográficas e prototipagem rápida para a fabricação de modelos médicos. Revista Brasileira de Engenharia Biomédica. 2003;19:103–15. [ ] 22. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. [ ] 23. Weng Y, Cao Y, Silva CA, et al. Tissue engineered composites of bone and cartilage for mandible condylar reconstruction. J Oral Maxillofac Surg. 2001;59:185–90. [ ] 24. Abukawa H, Terai H, Hannouche D, et al. Formation of a mandibular condyle in vitro by tissue engineering. J Oral Maxillofac Surg. 2003;61:94–100. [ ] 25. Williams JM, Adewunmib A, Schek RM, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–27. [ ] 26. Jakab K, Neagu A, Mironov V, et al. Organ printing: fiction or science. Biorheology. 2004;41: 371–5. [ ]
Received May 21, 2007. Accepted after revision August 20, 2007.
* Study developed at Universidade Federal de Santa Catarina (UFSC), Florianópolis, SC, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Universidade do Sul de Santa Catarina (Unisul), Tubarão, SC, and Centro de Pesquisa Renato Archer (CenPRA), Campinas, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554