Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 1 - Jan. /Feb. of 2008
Vol. 41 nº 1 - Jan. /Feb. of 2008
|
ORIGINAL ARTICLE
|
|
Dosimetric analysis of persons accompanying nuclear medicine patients in the therapeutic room |
|
|
Autho(rs): Jetro Pereira de Oliveira, Márcia Maria dos Santos Lopes, Luiz Antonio Ribeiro da Rosa, Léa Mirian Barbosa da Fonseca, Rossana Corbo |
|
|
Keywords: Nuclear medicine, Companion, Therapeutic room, Thermoluminescent dosimeter |
|
|
Abstract:
IFellow PhD degree, Department of Radiology, Division of Nuclear Medicine at Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), Rio de Janeiro, RJ, Brazil
INTRODUCTION Radiotherapy is based on the concept that iodine-131 (131I) is actively accumulated by a considerable part of a tumor and/or metastases originating from differentiated thyroid tumors, allowing the destruction or reduction of the tumor after receiving high radiation doses. The major difficulties are in selecting the patients for the effectiveness of the treatment, and the type of radiological protection to be adopted. A significant development has been observed in the field of radionuclide treatment in the last ten years, however, it is important to note that the therapy with radioactive iodine has been utilized since 1955(1). An example of nuclear medicine therapeutic procedure is the treatment of differentiated thyroid tumors (papillary and follicular). The rate of cure is high after radiotherapy, with a diseasefree survival of about 30 years. Even a patient affected by distant metastasis can keep the disease under long-term stable control(2). However, 131I is a source of high energy gamma radiation, leading to exposure of the environment around the patient. Therefore, the patients must be treated as an in-patient, in an appropriate room and in compliance with the guidelines for radiation protection established by Comissão Nacional de Energia Nuclear (CNEN) (National Commission for Nuclear Energy)(3,4). CNEN is a federal agency responsible for ensuring that facilities involved in the utilization of ionizing radiation operate in compliance with criteria and guidelines for radiation protection to guarantee that the radiation levels are as low as reasonably practicable aiming at minimizing the exposure to ionizing radiation of the population as a whole(5). The current criteria for isolation of nuclear medicine patients receiving therapeutic 131I doses of > 1,11 GBq require the admission of the patient to a hospital, in an individual therapeutic room. In case of necessity, two in-patients may be accommodated in a same therapeutic room, provided a mandatory protective barrier (lead-shielded screen) is placed between the beds. The therapeutic room, duly signalized and with controlled access, must have impermeable walls and floor to allow decontamination, rounded corners, private toilet and a lead-shielded screen beside the bed(3). The patients can be discharged as they present a 131I activity < 1.11 GBq(3), which corresponds to a radiation exposure rate of about 1.8 µC/kg, measured by a MIR series 7026 area monitor (Instituto de Engenharia Nuclear/CNEN), at a one-meter distance from the 131I source. The current legislation(4) establishes restricted doses, so it is unlikely that companions receive > 5 mSv during the whole patients' treatment period. Hypothyroidism and the isolation required for the treatment, most of times, result in significant psychological problems, so the procedure becomes extremely difficult for the patients. Many times, they cannot tolerate more than one hospital stay. In the case of disabled, psychologically disturbed patients or children, the presence of a companion is required, and both share the therapeutic room. Typically, the companion is a relative of the patient, and, preferably, he/she should not be in childbearing age or not intending to have a child. Both the patient and the companion are given instruction on radioprotection by the clinical staff, for example, the companion should approach the patient only as necessary to avoid unnecessary exposure to the 131I ionizing radiation present in the body of the patient. In case of a patient with thyroid cancer treated with 5550 MBq 131I, Mathieu et al.(6) report that the dose received by the companion for a 15-day period, after the patient is discharged, is on average 0.24 mSv. Coover et al.(7) report that patients are discharged immediately after receiving 131I with activities > 7400 MBq. From the psychological point of view, this new routine has been of great benefit for patients, their families and friends, besides minimizing the exposure to ionizing radiation of the whole clinical team, and reducing the treatment costs because of the early patients' discharge. The present study was aimed at evaluating the dose received during a two-day hospital stay by the companion of a patient submitted to treatment with 131I for thyroid cancer, considering that the companion and the patient share a same therapeutic room, and comparing the results obtained with the dose thresholds determined by the Brazilian radiological protection standards(3,4). The radiation dose was evaluated with the aid of a thermoluminescent dosimeter.
MATERIALS AND METHODS The primary objective of the present study was not evaluating a possible contamination of the companion, for example, by 131I inhalation, or skin contact with this radionuclide, but only evaluating the dose received by the companion because of the exposure to the radiation originating from the patient. Two experiments were developed to evaluate the received-doses in nuclear medicine patients' companions. In the first experiment, the companions were monitored with a thermoluminescent dosimeter, and in the second experiment, an acrylic phantom with the same type of dosimeter was utilized within the therapeutic room in the presence of the patient. The second experiment was adopted, considering that the presence of a companion is rare in these cases. This experiment allowed the obtention of a higher number of measurements, improving the statistical results accuracy. As the expected dose received by the companion was low, a single dosimeter was utilized for determining the mean dose for a determined administered 131I activity. Two of the most utilized 131I activities (3700 MBq and 5550 MBq) in Brazil were considered in the present study. The dose received by the companion of a patient who received a 3700 MBq activity could not be evaluated. However, all the doses involving this activity were obtained with the utilization of the phantom. Six companions of patients who had received 5550 MBq activities were monitored and the mean dose obtained in this situation corresponds to the quotient between the total integral dose (because of the six patients) and the number of patients. Ten patients were considered for both activities in the measurements with the phantom. Therefore, the values of mean doses for both 3700 MBq and 5550 MBq, correspond to the quotient between the total integral dose and the number of patients. All the measurements were performed with a lead-shielded screen placed between the beds of the patient and companion/phantom. All the individuals involved in the experiment were given explanations about the objectives of the study as well as about the procedures performed. Both patients and companions signed a term of free and informed consent. Age, sex and presence of other diseases were not taken into consideration for the individuals selection. The present study was submitted to and approved by the Committee for Ethics in Research of the Institution where the experiments were developed. The study presented a longitudinal character with a confidence level of 99% and three standard deviations. The experiment involved the evaluation of doses with thermoluminescent dosimeters. The phantom utilized was a polymethyl methacrylate (acrylic) cylinder, with 0.4 cm-thick walls (diameter = 8.0 cm and height = 13.0 cm) filled with water. Two thermoluminescent capsules were fixed to the cylinder surface with adhesive tape. The phantom remained on a bed at 2.85 m from the patient's bed during the whole treatment period (48 hours). The thermoluminescent powder utilized was lithium fluoride doped with magnesium, titanium and sodium (LiF:Mg,Ti,Na) produced by Philitech (France), under the code DTL937. This product is enriched with lithium-7 (7Li), and, considering the objectives of the present study, presents appropriate characteristics including low fading at ambient temperature (5%/year). The material regeneration was achieved by means of three-hour pre-irradiation thermal treatment at 450 ºC. The post-irradiation treatment was performed in the thermoluminescent reader at 125 C° for five seconds. This temperature is lower than the one for evaluating the termoluminescent dosimeters (440 ºC). An ETT oven (Fimel, France) was utilized in the thermal treatment of the dosimeters. The irradiated thermoluminescent powder was evaluated in an automatic Fimel PCL3 reader with a RTC XP1122 model photomultiplier. The thermoluminescent dosimeters were calibrated with the aid of a cobalt-60 source (60Co). The mean energy of the photons emitted by 131I is 364 keV and, for this energy, the DTL937 response energy dependence is not significant and can be disregarded in the dose calculation. Therefore, the calibration factor obtained for the 60Co gamma radiation energy can be utilized for determining the 131I doses without the necessity of additional correction. The thermoluminescent powder was enclosed into sealed, 2.3 cm long cylindrical polyethylene capsules with (external diameter = 0.5 cm). A pair of these capsules was externally fixed with an adhesive bandage to the cloth of the companion at the level of the chest. This site was chosen to facilitate the procedure reproducibility. The dosimeters remained in place during the whole treatment period, except during the bath. A 2.0 cm-thick lead-shielded screen (length = 1.07 m × height = 1.06 m) was placed between the patient and companion/phantom beds. At the end of the treatment, 48 hours after the iodine-131 ingestion by the patients, the dosimeters were removed from the companions clothes or phantoms for later analysis in the thermoluminescent dosimetry laboratory. The present study was developed in compliance with the Brazilian radiological protection standards(3,4). Additionally, the doses evaluated with the thermoluminescent dosimeters positioned on the phantom at 2.85 m from the patient were compared with the results of the calculation performed according to the procedures recommended by the North-American standards NRC-8.39 and 10CFR35.75(8,9). In this case, the lead-shielded screen was not utilized between the phantom and the patient to reproduce the calculation conditions described in those standards(8,9). Calculations and measurements were performed for a 131I activity = 5550 MBq (the most frequently utilized during treatments).
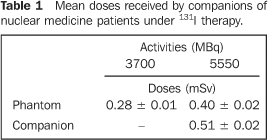
RESULTS Table 1 demonstrates that the integration of the doses measured by a pair of thermoluminescent dosimeter fixed to a phantom which had been exposed to ten patients who had received 3700 MBq activities of 131I, for a two-day treatment period allowed the determination of a mean dose of (0.28 ± 0.01) mSv. When the same phantom was submitted with another pair of dosimeters to other ten patients who had received 5550 MBq activities of 131I for a 24-hour period, this dosimeters recorded a mean integral dose of (0.40 ± 0.02) mSv. Also, Table 1 shows that a same pair of thermoluminescent dosimeters recorded a mean integral dose of (0.51 ± 0.02) mSv, when fixed to the clothes of six persons accompanying patients who had ingested 5550 MBq activities of 131I, sharing a same therapeutic room for a two-day period.
The maximum dose calculated according to the North-American standards NRC-8.39 and 10CFR35.75(8,9), for a determined point at 2.85 m from a point source of 5550 MBq of 131I, is 0.42 mSv. The three values recorded by thermoluminescent dosimeters fixed to the phantom and placed at the same 2.85 m-distance from patients who had ingested the same activity of 131I (5550 MBq), where: (1.24 ± 0.09) mSv, (0.98 ± 0.07) mSv and (0.99 ± 0.07) mSv, corresponding to a mean value of (1.07 ± 0.13) mSv.
DISCUSSION The difference observed between mean dose values found in the companions and in the phantom sharing a same therapeutic room with patients who received 5550 MBq of 131I, can be explained by the deambulation of the companion and consequential variation in distance of his/her dosimeter from the patient, while the dosimeters fixed on the phantom remain static on the other bed. As expected, the greater the administered 131I activity, the higher the dose recorded by the thermoluminescent dosimeter. It is important to emphasize that all the doses measured are below the radiation dose threshold established by the radioprotection standard NN-3.01(4), corresponding to 5 mSv/year. Therefore, according to the mentioned standards, from the radiological protection point of view, there is no impediment to any person accompanying a patient under treatment with 131I for thyroid cancer, receiving activities ranging between 3700 MBq and 5550 MBq. The fact that the dose measured in the phantom (1.08 ± 0.03) mSv was higher than the calculated dose (0.42 mSv) is explained by the fact that the source was not a point source, considering that the radionuclide is distributed throughout the body of the patient whose movements can reduce the 2.85 m-distance considered for calculation of the dose to the point source. It is important to emphasize that, even in the absence of the lead-shielded screen, both the measured and calculated values are below the 5 mSv threshold established both by the Brazilian(3,4) and North-American standards(8,9). The results of the present study confirm that the radiation dose received by a companion sharing a same therapeutic room with a nuclear medicine patient who has been given 3700 MBq or 5550 MBq of iodine-131 during a two-day period does not exceed (0.51 ± 0.02) mSv. Therefore, one can conclude that, under the radioprotection point of view, the dose received by such person is in compliance with the Brazilian standards(3,4), establishing a radiation dose threshold of 5 mSv/year. These results are in agreement with the results reported by Mathieu et al.(6), and Coover et al(7). Additionally, according to the results of the present study and those reported by Mathieu et al.(6), the companion of a patient who received 5550 MBq of 131I will receive about 0.75 mSv over a 17-day period, corresponding to a two-day treatment period (0.51 mSv) plus 15 days following the patient discharge from the hospital (0.24 mSv). Considering that the patient and his/her companion share a same therapeutic room for a twoday period, the companion contamination with 131I administered to the patient is natural. 131I is a volatile radionuclide which is released through the respiration, skin pores, saliva, feces and urine of the patient. However, as previously mentioned, the present study has not contemplated the evaluation of the dose received by companions as a result of a possible contamination. Notwithstanding, the results obtained both for the companions (0.51 ± 0.02) mSv and for the phantom (0.40 ± 0.02) mSv do not seem to indicate a significant companions contamination, if this has really occurred. Acknowledgements The first author thanks the Coordenação de Aperfeiçoamento de Pessoas de Nível Superior (Capes) for the financial support, and all the persons involved in the development of the present study, as well as Ana Maria de Oliveira Rebelo, radioprotection supervisor for the Department of Nuclear Medicine at Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro, and Arnaldo Rangel Carvalho, technician in the Thermoluminescent Dosimetry Laboratory at Instituto de Radioproteção e Dosimetria – Comissão Nacional de Energia Nuclear.
REFERENCES 1. Chapman EM, Maloof F. The use of radioactive iodine in the diagnosis and treatment of hyperthyroidism: ten years' experience. Medicine (Baltimore). 1955;34:261–70. [ ] 2. Brucer M. Thyroid radioiodine uptake measurement. A standard system for universal intercalibration. USAEC Report. ORINS 1959;19. [ ] 3. CNEN-NE-3.05. Requisitos de radioproteção e segurança para serviços de medicina nuclear. Comissão Nacional de Energia Nuclear. [Acessado em: 28/2/2007]. Disponível em: http://www. cnen.gov.br/seguranca/normas/mostra-norma. asp?op=305 [ ] 4. CNEN-NN-3.01. Diretrizes básicas de proteção radiológica. Comissão Nacional de Energia Nuclear. [Acessado em: 28/2/2007]. Disponível em: http://www.cnen.gov.br/seguranca/normas/mostra-norma.asp?op=301 5. Mendes LCG, Fonseca LMB, Carvalho ACP. Proposta de método de inspeção de radioproteção aplicada em instalações de medicina nuclear. Radiol Bras. 2004;37:115–23. [ ] 6. Mathieu I, Caussin J, Smeesters P, et al. Recommended restrictions after 131I therapy: measured doses in family members. Health Phys. 1999;76: 129–36. [ ] 7. Coover LR, Silberstein EB, Kuhn PJ, et al. Therapeutic 131I in outpatients: a simplified method conforming to the Code of Federal Regulations, title 10, part 35.75. J Nucl Med. 2000;41:1868–75. [ ] 8. U.S. Nuclear Regulatory Commission. Regulatory Guide 8.39. Release of patients administered radioactive materials. Washington, DC: Office of Nuclear Regulatory Research; 1997. p.14–9. [ ] 9. The Code of Federal Regulations: title 10, energy; part 35. Medical use of byproduct material; section 75, release of individuals containing radiopharmaceuticals or permanent implants. Fed Regist. 1997;62:4133. [ ]
Received March 23, 2007. Accepted after revision July 10, 2007.
* Study developed in the Division of Nuclear Medicine at Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554