Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 2 - Mar. / Apr. of 2009
Vol. 42 nº 2 - Mar. / Apr. of 2009
|
ORIGINAL ARTICLE
|
|
Changing trends in the treatment of Graves' disease with radioiodine: a 12-year experience in a university hospital |
|
|
Autho(rs): Marcus Vinicius Leitão de Souza, Honomar Ferreira de Souza, Alexandru Buescu, Mario Vaisman |
|
|
Keywords: Graves' disease, Methimazole, Sodium iodide |
|
|
Abstract:
IMaster, Endocrinologist at Instituto Estadual de Diabetes e Endocrinologia (IEDE), Rio de Janeiro, RJ, Brazil
INTRODUCTION More than 60 years have passed since the first description of radioactive iodine in the treatment of hyperthyroidism, in 1941 at the Massachusetts General Hospital in Boston, the isotope NaI-130I being utilized at that time(1,2). Because of the low cost, higher effectiveness in the thyroid cells destruction, and eight-day half-life, NaI-131I has become the isotope of choice and is still widely utilized nowadays. However, antithyroid drugs have been the first option for treating hyperthyroidism, particularly Graves' disease, not only in Brazil but also in the whole South America(3), principally aiming at achieving the disease remission. Considering the mean cost of treatment and the mean rate of remission after 12-24 months of treatment (approximately 50-60%)(4-9), the early indication for a definite treatment with NaI-131I (therapeutic dose - TD) should be a more frequent option. Issues regarding the safety of NaI-131I with emphasis on female patients and a possible effect of this isotope on their fertility have represented a hindrance to the early referral of patients for treatment. Aiming at evaluating the possible changes in the profile of patients referred for TD, as well as the change in the conduct related to the administered dose and the consequential effect on the rate of cure of these patients, the authors collected data in the Unit of Nuclear Medicine of Hospital Universitário Clementino Fraga Filho - Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), in a 12-year period between January 1990 and December 2001. The evaluation of the TD effectiveness as well as the influence of the change in the TD calculation was based on the analysis of the thyroid functional status one year after the TD.
MATERIALS AND METHODS A retrospective study was developed with analysis of medical records of patients submitted to TD in the Unit of Nuclear Medicine of HUCFF-UFRJ, in the period from January 1990 to December 2001. The NaI-131I doses calculation was based on the thyroid volume observed at clinical examination, the 24-hour NaI-131I uptake, the hyperthyroidism severity and the eventual presence of comorbidities. Doses ranged from 50-150 μCi/g of thyroid tissue, but no report was found onthe values of μCi/g thyroid tissue defined for each of the periods covered by the present study. Considering that the mentioned period is a continuous variable and, for the purposes of comparative analysis, the 12 years were segmented into four-year periods as follows: January 1990 to December 1993 (period 1), January 1994 to December 1997 (period 2), and January 1998 to December 2001 (period 3). The present study included patients submitted to TD, with decompensated hyperthyroidism (decreased TSH level and increased T4 level) in association with signs and symptoms of hyperthyroidism, with or without previous treatment with antithyroid drugs. Pre-TD laboratory test included the latest hormone dosage and therefore the hormone level reflecting the degree of hyperthyroidism decompensation in spite of antithyroid therapy. The sample uniformity was achieved through exclusion of patients based on the following criteria: patients submitted to thyroidectomy previously to the TD; patients previously submitted to TD; patients submitted to TD for multinodular toxic goiter or single toxic nodule undergoing antithyroid therapy for less than three months before TD; patients submitted to TD and undergoing antithyroid therapy; patients without any hormone dosage in the 9-12 month post-TD interval; patients with no pre-TD clinical and laboratory data. The following variables were included for the purposes of statistical analysis: age, sex, time of antithyroid therapy, goiter volume, pre-TD 24-hour NaI-131I uptake, pre-TD hormone levels, dose of NaI-131I delivered, type of antithyroid drug, antithyroid drug dose, post-TD (9-12 months) hormone levels with classification of the functional thyroid status in this period (cured or non-cured). Antithyroid drug dose corresponded to the dose received by the patient at the time of the pre-TD withdrawal. Only patients undergoing antithyroid therapy for at least three months could be included in the group with pre-TD antithyroid therapy. Patients without antithyroid drugs for more than three months were considered as without pre-TD antithyroid therapy. The goiter size was determined through clinical evaluation and graded from 0 to 3 as follows: 0 = absent goiter, 1 = one- to two-fold increased thyroid, 2 = more than two- to four-fold increased thyroid, and 3 = more than four-fold increased thyroid. Laboratory tests for hormone dosage included free T4 (ng/dl), TSH (mU/l), total T4 (μg/dl) and total T3 (ng/dl). In the post-TD 9-12-month period the patients were re-evaluated for hormone dosage and classified into two different groups as follows: a) Cured patients: with normal free T4 levels (euthyroid patients); with normal free T4 levels using levothyroxine (treated hyperthyroid patients); low free T4 levels with or without levothyroxine (hypothyroid or compensated patients). b) Non-cured patients: with high free T4 levels without any medication; with high free T4 levels using antithyroid drugs; normal free T4 levels under antithyroid therapy. Patients with high free T4 levels under levothyroxine therapy as well as those with low free T4 levels under antithyroid therapy were classified according to their progression along the follow-up. TSH serum levels were not taken into consideration for this classification because the thyroid axis suppression may extend for a long period even after the clinical euthyroid status is achieved(10,11). In patients whose free T4 level was not available, the total T4 level was considered according to the same, above mentioned criteria. Statistical analysis The software Epi-Info version 6.0 was utilized for statistical analysis. The Kruskal-Wallis test was utilized for comparison of numerical and category variables among age, time of antithyroid therapy, free T4 levels, total T4 levels, total T3 levels, NaI-131I uptake and therapeutic dose in the three periods determined for the present study. The chi-square (χ2) test was utilized for bivariate analysis with comparison of two category variables among sex, goiter volume (segmented from 0 to 4), type of antithyroid drug utilized and hyperthyroidism remission or not one year after after TD in the three mentioned periods, with values > 3.84 corresponding to p< 0.05. Statical significance was determined at 5% or p < 0,05.
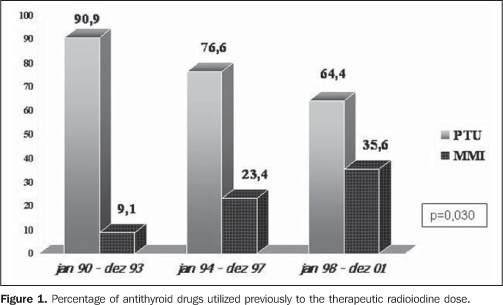
RESULTS Among 774 dossiers of patients with hyperthyroidism submitted to TD, 226 which met both the mentioned inclusion and exclusion criteria were analyzed. All the results are shown on Table 1. Over the years, a significant increase was observed in the number of patients referred for TD (27, 70 and 129 in the first, was similar over the years and third periods, respectively). Over the years, the clinical profile of the patients referred for TD remained stable as reflected by the statistical similarity of data regarding goiter volume, hormone levels (free T4, total T3 and TSH) pre-TD iodine uptake as well as the pre-TD antithyroid drug dose. Statistically significant changes in relation to the clinical profile of the patients are reflected by the following aspects: 1) a significant increase in the number of female patients referred for TD, particularly as from 1993 (62.9%, 82.8% and 86.8% respectively in the first, second and third periods, corresponding to p = 0.005); 2) type of pre-TD antithyroid therapy, with a significant increase in the number of patients utilizing methimazole (9.1%, 23.4% and 35.6% respectively in the first, second and third period, with p = 0.03), as demonstrated on Figure 1.
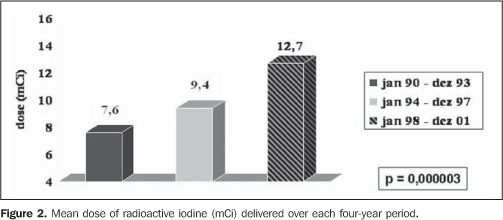
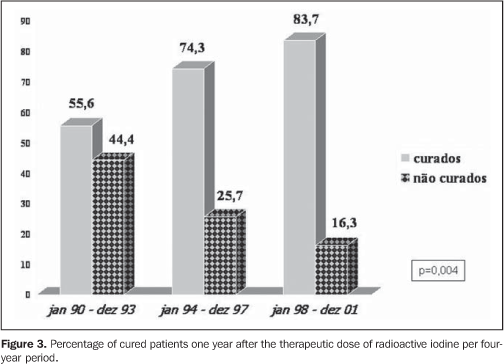
The radioiodine dose delivered presented a quite significant increase (7.6, 9.4 and 12.7 mCi, respectively in the first, second and third periods, with p = 0.000003), as shown on Figure 2, affecting directly the percentage of patients cured of hyperthyroidism one year after the TD (55.6%, 74.3% and 83.7%, respectively in the first, second and third periods, with p = 0.004) (Figure 3).
In a separate analysis of patients cured one year after the DT, no significant change in the percentage of hyperthyroid patients was observed with 9/15 (60%), 36/52 (69%) and 67/108 (62%) respectively in the first, second and third periods.
DISCUSSION The ideal therapy for hyperthyroidism is still to be established, and the three classical therapeutic approaches - namely, surgery, antithyroid therapy or TD of NaI-131I - present both advantages and disadvantages(12-15). The criteria for administration of radioactive iodine as a treatment for hyperthyroidism in Graves' disease still lack uniformity through different countries, especially in relation to the administered dose, moment of administration and which factors might affect the treatment effectiveness. In the USA, there is a remarkable preference for TD, with early indication of this type of therapy(16). According to scientific citations, the dose per gram of thyroid tissue has been progressively increased in order to prevent recidivation or even minimizing chances of non-remission with necessity of repetition of the TD(17). In the present study the authors observed that the number of patients referred for TD more than duplicated at each period, reflecting its higher acceptance as an excellent method for definite treatment, besides the therapy safety(12,17). The influence of the North American literature, which reports the indication of radioactive iodine in up to 69% of cases of hyperthyroidism as the initial method of treatment(16), may also be an associated factor. As regards the mean pre-TD antithyroid therapy time, there was a subtle decrease over the years, although without any significant difference (p = 0.289), maybe suggesting a trend to an earlier indication for a definite therapy. As regards the utilization of pre-TD antithyroid therapy, there was a significant increase in the number o patients treated with methimazole (Figure 1), maybe reflecting the results of some studies suggesting a more pronounced radioprotector effect of propylthiouracil(18-22), besides the major facility for a pre-TD methimazole withdrawal(23). Another interesting finding was the increase in the percentage of female patients referred for TD, with a 60.7% female prevalence in the period between 1990 and 1993, increasing to 83% in the 1994-1997 period and remaining stable (86.3%) in the 1998-2001 period. These data reflect not only the TD safety, especially for young patients with Graves' disease, as demonstrated by Metso et al. in a recent population study with 2,793 patients(24), but re-emphasizing the radioactive iodine safety even for women in childbearing age, as already demonstrated by Brandão et al.(25), considering that, among the 189 women included in the study, 40.7% (n = 77) were 16-40-year old, and 26.7% (n = 30) were 16-30 year old. A crucial issue to be discussed is the difference in the radioiodine doses (increase from 7.6 mCi to 9.4 mCi and 12.7 mCi, with p = 0.000003). Much has been tried to define an ideal dose(17,26), from low, fixed doses(27,28) and dose calculations(12) to achieve euthyroidism, to high fixed doses(29,30), aiming at guaranteeing the hypothyroidism and, consequently, the "cure" of hyperthyroidism. It is clearly demonstrated that the higher the dose, the higher the rate of hypothyroidism; and the lower the dose, the higher the chance of recidivation. Dose calculations, where utilized, are extremely variable (75-200 μCi/g) (12,17), even at a same health center, especially in relation to goiter volume, hyperthyroidism severity and presence of cardiopathy.The utilization of ultrasonography in the measurement of goiter volume is of little practical significance and does not make any difference in the final effect of TD as compared with the clinical evaluation(31). Although it is seemingly non-practical, a simple methodology was recently described for biokinetic evaluation of radioactive iodine as well as the evaluation of the absorbed dose/administered activity ratio(32), that might be useful for more effective dose calculations. However, this methodology has not been routinely adopted yet. So, the currently adopted doses have been increasingly higher, particularly when the eventual presence of comorbidities and possible risks for patients (cardiopathy being the most feared one) are taken into consideration. Upon all these considerations, one can clearly observe the increase in calculated doses over the years aiming at guaranteeing a cure of hyperthyroidism as fast as possible, even at the expense of a relatively early onset of hypothyroidism. Patients with not so severe hyperthyroidism, without comorbidities and with good indicators for post antithyroid therapy remission cannot be forgotten(5,33), to give them a chance of euthyroid independently from the utilization of any medication. So, it seems to be reasonable to observe all the indicators of Graves’ disease severity and discussing with her/him the best option for treatment. Finally, as well as Hernández-Jiménez et al.(34), we could observe that the linear increase in the administered dose (Figure 2) corresponded to a progressive increase in the percentage of cured patients (Figure 3) - from 55% in the first period, to 74% in the second and, finally 83% in the third period (p = 0.004). The authors concluded that in their health unit, the number of patients referred for TD presented a quite significant increase, which corroborates the endocrinologists' acceptance of radioactive iodine as a safe method for definite treatment for Graves' disease. The administered radioiodine dose presented a significant increase in over the three periods covered by the present study coincidentally with a significant decrease in the percentage of non-cured patients one year after the TD. Additionally, it may be concluded that the patients have not been referred earlier for TD, and that the utilization of methimazole has increased in patients who are to be submitted to radioiodine therapy, maybe because of the very probable propylthiouracil radioprotector effect already confirmed by prospective controlled studies(20,21). Finally, a significant percent increase was observed in the female group (even women in childbearing age) referred for TD, approaching the actual female prevalence of Graves' disease.
REFERENCES 1. Becker DV, Sawin CT. Radioiodine and thyroid disease: the beginning. Semin Nucl Med. 1996; 26:155-64. [ ] 2. Sawin CT, Becker DV. Radioiodine and the treatment of hyperthyroidism: the early history. Thyroid. 1997;7:163-76. [ ] 3. Romaldini JH. Case selection and restrictions recommended to patients with hyperthyroidism in South America. Thyroid. 1997;7:225-8. [ ] 4. Bonnema SJ, Bartalena L, Toft AD, et al. Controversies in radioiodine therapy: relation to ophthalmopathy, the possible radioprotective effect of antithyroid drugs, and use in large goiters. Eur J Endocrinol. 2002;147:1-11. [ ] 5. Vitti P, Rago T, Chiovato L, et al. Clinical features of patients with Graves' disease undergoing remission after antithyroid drug treatment. Thyroid. 1997;7:369-75. [ ] 6. Allannic H, Fauchet R, Orgiazzi J, et al. Antithyroid drugs and Graves' disease: a prospective randomized evaluation of the efficacy of treatment duration. J Clin Endocrinol Metab. 1990;70:675-9. [ ] 7. Hedley AJ, Young RE, Jones SJ, et al. Antithyroid drugs in the treatment of hyperthyroidism of Graves' disease: long-term follow-up of 434 patients. Scottish Automated Follow-Up Register Group. Clin Endocrinol (Oxf). 1989;31:209-18. [ ] 8. Sugrue D, McEvoy M, Feely J, et al. Hyperthyroidism in the land of Graves: results of treatment by surgery, radio-iodine and carbimazole in 837 cases. Q J Med. 1980;49:51-61. [ ] 9. Tamai H, Nakagawa T, Fukino O, et al. Thionamide therapy in Graves' disease: relation of relapse rate to duration of therapy. Ann Intern Med. 1980;92: 488-90. [ ] 10. Allahabadia A, Daykin J, Sheppard MC, et al. Radioiodine treatment of hyperthyroidism - prognostic factors for outcome. J Clin Endocrinol Metab. 2001;86:3611-7. [ ] 11. Benker G, Reinwein D, Kahaly G, et al. Is there a methimazole dose effect on remission rate in Graves' disease? Results from a long-term prospective study. Clin Endocrinol (Oxf). 1998;49: 451-7. [ ] 12. Cooper DS. Treatment of thyrotoxicosis. In: Braverman LE, Utiger RD, editors. Werner & Ingbar's The thyroid: a fundamental and clinical text. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 691-715. [ ] 13. Sosa JA, Bowman HM, Tielsch JM, et al. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228:320-30. [ ] 14. Hermann M, Roka R, Richter B, et al. Reoperation as treatment of relapse after subtotal thyroidectomy in Graves' disease. Surgery. 1999;125:522-8. [ ] 15. Törring O, Tallstedt L, Wallin G, et al. Graves' hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine - a prospective randomized study. Thyroid Study Group. J Clin Endocrinol Metab. 1996;81:2986-93. [ ] 16. Levy EG. Treatment of Graves' disease: the American way. Baillières Clin Endocrinol Metab. 1997;11:585-95. [ ] 17. Kaplan MM, Meier DA, Dworkin HJ. Treatment of hyperthyroidism with radioactive iodine. Endocrinol Metab Clin North Am. 1998;27:205-23. [ ] 18. Imseis RE, Vanmiddlesworth L, Massie JD, et al. Pretreatment with propylthiouracil but not methimazole reduces the therapeutic efficacy of iodine-131 in hyperthyroidism. J Clin Endocrinol Metab. 1998;83:685-7. [ ] 19. Tuttle RM, Patience T, Budd S. Treatment with propylthiouracil before radioactive iodine therapy is associated with a higher treatment failure rate than therapy with radioactive iodine alone in Graves' disease. Thyroid. 1995;5:243-7. [ ] 20. Santos RB, Romaldini JH, Ward LS. Propylthiouracil reduces the effectiveness of radioiodine treatment in hyperthyroid patients with Graves' disease. Thyroid. 2004;14:525-30. [ ] 21. Bonnema SJ, Bennedbaek FN, Veje A, et al. Propylthiouracil before 131-I therapy of hyperthyroid diseases: effect on cure rate evaluated by a randomized clinical trial. J Clin Endocrinol Metab. 2004;89:4439-44. [ ] 22. de Souza MV, Buescu A, Vaisman M, et al. The effect of propylthiouracil on the efficacy of radioiodine (I-131) therapy in Graves' hyperthyroidism. Arq Bras Endocrinol Metab. 2006;50:1088-95. [ ] 23. Andrade VA, Gross JL, Maia AL. The effect of methimazole pretreatment on the efficacy of radioactive iodine therapy in Graves' hyperthyroidism: one year follow-up of a prospective, randomized study. J Clin Endocrinol Metab. 2001;86: 3488-93. [ ] 24. Metso S, Jaatinen P, Huhtala H, et al. Increased cardiovascular and cancer mortality after radioiodine treatment for hyperthyroidism. J Clin Endocrinol Metab. 2007;92:2190-6. [ ] 25. Brandão CDG, Antonucci J, Correa ND, et al. Efeitos da radioiodoterapia nas gerações futuras de mulheres com carcinoma diferenciado de tireóide. Radiol Bras. 2004;37:51-5. [ ] 26. Nordyke RA, Gilbert FI Jr. Optimal iodine-131 dose for eliminating hyperthyroidism in Graves' disease. J Nucl Med. 1991;32:411-6. [ ] 27. Cevallos JL, Hagen GA, Maloof F, et al. Low-dosage 131-I therapy of thyrotoxicosis (diffuse goiters). A five-year follow-up study. N Engl J Med. 1974;290:141-3. [ ] 28. Sridama V, McCormick M, Kaplan EL, et al. Long-term follow-up study of compensated low-dose 131I therapy for Graves' disease. N Engl J Med. 1984;311:426-32. [ ] 29. Eriksson E, Eriksson K, Wahlberg P. Treatment of hyperthyroidism with standard doses of radioiodine aiming at ablation. Acta Med Scand. 1985; 217:55-60. [ ] 30. Safa AM, Skillern PG. Treatment of hyperthyroidism with a large initial dose of sodium iodide I-131. Arch Intern Med. 1975;135:673-5. [ ] 31. Tsuruta M, Nagayama Y, Yokoyama N, et al. Long-term follow-up studies on iodine-131 treatment of hyperthyroid Graves' disease based on the measurement of thyroid volume by ultrasonography. Ann Nucl Med. 1993;7:193-7. [ ] 32. Araujo F, Melo RC, Rebelo AMO, et al. Proposta de metodologia para tratamento individualizado com iodo-131 em pacientes portadores de hipertireoidismo da doença de Graves. Radiol Bras. 2007;40:389-95. [ ] 33. Cooper DS. Antithyroid drugs in the management of patients with Graves' disease: an evidence-based approach to therapeutic controversies. J Clin Endocrinol Metab. 2003;88:3474-81. [ ] 34. Hernández-Jiménez S, Pachón-Burgos A, Aguilar-Salinas CA, et al. Radioiodine treatment in autoimmune hyperthyroidism: analysis of outcomes in relation to dosage. Arch Med Res. 2007;38: 185-9. [ ] Received September 3, 2008. * Study developed in the Service of Endocrinology, Department of Medical Sciences at Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554