Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 1 - Jan. /Feb. of 2009
Vol. 42 nº 1 - Jan. /Feb. of 2009
|
ORIGINAL ARTICLE
|
|
Magnetic resonance spectroscopy imaging in the diagnosis of prostate cancer: initial experience |
|
|
Autho(rs): Homero José de Farias e Melo, Denis Szejnfeld, Cristiano Silveira Paiva, Nitamar Abdala, Homero Oliveira de Arruda, Suzan Menasce Goldman, Jacob Szejnfeld |
|
|
Keywords: Magnetic resonance spectroscopy imaging, Prostate, Prostatic neoplasm |
|
|
Abstract:
IMaster, Fellow PhD degree in Radiological Sciences at Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil
INTRODUCTION The increase in the incidence of prostate cancer from 86,000 cases in 1985 to 218,890 cases in 2007 in the United States alone, has transformed this disease not only into an important medical problem, but also into a public health and socioeconomic(1,2) one. In Brazil, 49,530 new cases are estimated in 2008, according to Instituto Nacional de Câncer(3). However, in spite of the fact that digital rectal examination is considered as the first diagnostic tool, it is limited as it presents negative results for non palpable nodules (T1c stage)(4). The uncertainty with respect to the upper limit value for cancer screening with prostate specific antigen (PSA) and its low specificity contribute for two current clinical challenges: only one in four men with PSA > 4 ng/ml actually present cancer at biopsy, and approximately one third of the prostate cancers are detected in men with normal PSA(5). Ultrasonography is widely utilized because of its relatively low cost, and when used with the transrectal probe this method offers the best opportunity to guide the gland biopsy(6,7) . However it is limited to local staging due to the difficulty in the early diagnosis of the extracapsular extent and high operator dependency, which limits the reproducibility of the technique(8,9) Amongst the other radiological techniques, magnetic resonance imaging (MRI) is the most useful diagnostic tool for evaluation of tumor stages, mainly on cases where endorectal coil is utilized(8) . Magnetic resonance imaging has a significantly higher sensitivity (51-89%) in the detection of the tumor when compared with transrectal ultrasonography (TRUS) (27-86%). However, both methods present a low specificity (58-94%)(8,9). Recently, magnetic resonance spectroscopy (MRS) brought a new diagnostic hope. Based on anatomical data generated by MRI, this method can demonstrate the metabolic indicators detected in the prostate gland, enhancing the accuracy in the probable localization of the tumor(8,10-14). In Brazil, there are still only few MRI centers with the technical background required to perform prostate examinations with spectroscopy. Additionally, this technique is not covered by health insurance plans or by the Sistema Único de Saúde (SUS) (Brazilian Public Health System). With the real perspective of a significant improvement in prostate cancer diagnosis utilizing MRSI, a protocol for the acquisition of spectroscopic data was implemented at the Department of Imaging Diagnosis - Universidade Federal de São Paulo/Escola Paulista de Medicina (Uni-fesp/EPM).
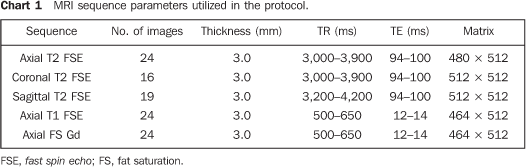
MATERIALS AND METHODS The present prospective study was developed between February of 2004 and December of 2005, with 41 male patients with ages ranging from 51 to 80 years, and mean age of 67 years, selected by the Nucleus of Prostate Research (Nuppro) of Unifesp/EPM. Throughout this period, Nuppro assisted approximately 2,000 patients. The patients were divided into two groups: group A - patients with clinic-laboratory diagnostic suspicion of cancer, including 28 patients selected due to the fact that they had one or more negative biopsies, and persistently high PSA levels and/or altered digital prostate examination results; group B - patients with confirmed diagnosis of prostate cancer, including 13 patients selected due to the fact of having positive biopsies (one Gleason 3, one Gleason 5, three Gleason 6, six Gleason 7, two Gleason 8). The patients from group A with diagnostic suspicion presented indication for prostate biopsies because of persistently high PSA levels. For these patients, MRSI was performed in order to identify the possible altered areas to be approached at US guided biopsy. In the case of Group B patients, besides the identification of tumor site, locoregional staging of the prostate cancer was performed by MRI. Patients included in this group were not submitted to biopsy after MRSI, as the previously performed biopsies were positive. This study protocol was previously submitted to and approved by the Committee for Ethics in Research of Unifesp/EPM. All patients signed a term of free and informed consent. Examination protocol Preparation and positioning of the patient The patients preparation for examination consisted of four-hour fasting and intravenous administration of antispasmodic drug. No intestinal lavage was performed. All patients were previously instructed on the examination procedures. The endorectal coil introduction was performed with the patient in left lateral decubitus. The coil characteristics indicate that the blue line on its shaft should be positioned towards the ventral direction in relation to the patient, and the first portion of the shaft should be positioned at the level of the anal border. The extremity of the endorectal coil was protected with an unlubricated condom externally lubricated with Xylocaine® gel. Then, the balloon at the end of the coil was inflated with 100 ml of air to distend the rectum wall, keeping the safety support to avoid displacement of the coil and loss of the condom. After completion of this operation, the patient was slowly positioned in dorsal decubitus, holding the coil shaft. The positioning consisted in connecting endorectal, phased-array and column coils (SP's), with the objective of optimizing the image acquisition and spectroscopy. With these maneuvers, the patient remained in dorsal decubitus, with the feet entering the equipment first, and with the arms towards the floor. Finally, the patient was asked to remain still during the whole examination process, breathing normally, and without contracting the rectal channel. Exam technique Protocol for magnetic resonance image acquisition All examinations were performed in a 1.5 T Magnetom Sonata unit with a gradient of 43 mT/m (Siemens Medical Systems; Erlangen, Germany), at the Department of Imaging Diagnosis of Unifesp/EPM. The radiofrequency body coil, present in the equipment itself, was utilized for excitation, the endorectal coil, combined with the matrix coil, in the prepubic region of the patient; the SP's, located in the presacral region of the patient, were utilized for MR signal reception. The MRI examination programming was performed as recommended in the literature, as demonstrated on Figure 1. Chart 1 includes a summary of the sequence parameters applied.
In the sagittal plane, the positioning was performed following the longest axis of the prostate, aligning the pubic symphysis with the lumbar spine. In the coronal plane, the block was angled according to the longest axis of the prostate. In the axial plane, the angle was according to the longest prostate axis, in such a manner to position the images from the pubic symphysis to the end of the seminal vesicles. Paramagnetic contrast injection (10 ml) was systematically performed in the patients, after spectroscopy, on T1-weighted sequences with fat saturation. Protocol for spectroscopic data acquisition A multiple-volume system was utilized in the selection of the spectroscopic volume of interest, acquired by the commercially available sequence PRESS CSI 3D hybrid (TR 1,300 ms/TE 120 ms; FOV 60-100 cm2; voxel 0.10-0.22 cm3, 4-5 acquisitions) (Siemens Medical Systems; Erlangen, Germany), so as to minimize possible artifacts of the periprostatic structures. The MRSI programming included T2-weighted sequences so as to evaluate the whole prostatic volume as shown on Figure 2. Besides being freely angled, without any limitations for the spectroscopic acquisition, the MRSI sequence offered the possibility of using eight external saturation bars, thus minimizing the effects of the non homogenization of the field by the effect of magnetic susceptibility, originated from the air within the coil, bone structures, periprostatic fat, and presence of urine in the bladder and in the penile urethra. For the prostate spectroscopy, spectral suppression was utilized both for water and fat, according to recommended in literature(8,10-13,15-18) , making it possible for the lipids present in the prostate, not to interfere in the acquisition. The total examination time, including the patients positioning, MR image and spectroscopic data acquisition, was approximately 45 minutes. Image and spectroscopic data analysis The studies were consensually evaluated by two observers with regards to the morphology, and by one observer with respect to the spectral analysis. The first phase consisted of the sequences analysis for evaluation of the prostate morphology, areas of signal alteration (peripheral zone and transition zone), identification of possible blood residues, evaluation of periprostatic fat and seminal vesicle integrity. The second phase consisted of the analysis of spectroscopy acquisition. The postprocessing was made in a Leonardo® workstation (Siemens Medical Systems; Erlangen, Germany). The spectroscopic data were measured after baseline correction, of the chemical deviation, with water as reference and T2-weighted in the three orthogonal planes to evaluate the positioning of the voxel. The Fourier transformation was prioritized in three spatial directions, applying the Hamming filter. The whole prostatic volume was qualitatively evaluated. Initially, the regions of interest were identified as those that presented an increase in choline levels and decreased citrate levels, therefore nominated target areas. Then a qualitative and quantitative evaluation was performed, identifying the relationship between metabolite peak amplitude ratios target area. Upon conclusion of the two phases, the analyses results were compared in consensus and classified into three groups: 1 - areas detected by MRI in agreement with MRSI; 2 - areas detected by MRI in disagreement with MRSI; 3 - areas not detected by MRI but characterized by MRSI. Biopsy of the target area Based on MRI and MRSI results, TRUS-guided biopsies were performed in the Ultrasonography Sector of the Department of Imaging Diagnosis at Unifesp/EPM, utilizing a Philips SD 800 unit (Philips Medical Systems; Eindhoven, The Netherlands) and 18 G needle. The radiologists were informed on the position of the target areas according to the McNeal's nomenclature(19). In patients with no suspect areas at MRI and MRSI, randomized biopsies were performed obtaining 18 fragments. Statistical analysis A descriptive study was developed, considering the small number of patients. Even with this limitation, one tried to demonstrate the most relevant findings by association measurements (sensitivity, specificity, accuracy, positive predictive value and negative predictive value).
RESULTS In relation to alterations identified at MRSI and /or MRI, compared with biopsies after MRSI in the 28 patients included in group A (high PSA levels and negative biopsies), 12 presented alterations both at MRI and MRSI , 8 only at MRSI , in 7 no alteration was observed, and in one, alteration was observed only at MRI. In group A, the relationship between citrate/choline ratio (positive: 0.221 ± 0.166; negative: 1.441 ± 0.562) and (choline + creatine)/citrate ratio (positive: 7.922 ± 4.976; negative: 2.151 ± 1.089) amplitudes was established. These data were measured in a systematic manner, utilizing the same analysis protocol in the target area of all the patients included in the present study. In the group A, for patients with alterations both at MRI and MRSI, presented 100% sensitivity, 47% specificity, 58% accuracy, 37% positive predictive value, and 100% negative predictive value (Figure 3). All of the 13 patients from group B presented alterations both at MRI and MRSI. Citrate/choline ratio (mean: 0.369 ± 0.231) and (choline+creatine)/citrate ratio (mean: 5.471 ± 4.355) amplitudes of group B were measured in a systematic manner, with the same analysis protocol, in the area affected by cancer of all the patients included in the present study. For the group B, sensitivity, specificity, accuracy, positive predictive value and negative predictive value were 100%.
DISCUSSION Prostate cancer screening fundamentals are based on the fact that patients diagnosed at screening tend to present a more favorable stage as compared with those clinically diagnosed, with a possible decrease in the rate of specific mortality due to prostate cancer. Magnetic resonance imaging is commonly utilized for the tumor staging after a diagnosis is established by prostatic biopsy. When the disease is confined to the prostate, the capsule will appear intact, even if there is an extensive contact or regular bulging between the capsule and the tumor(8,20). Additionally, MRI can also demonstrate the prostate anatomy, identifying areas with alteration of signal intensity, which may represent focal lesions in the gland. Thus, this method provides an extensive evaluation of patients with prostate cancer, for its capacity of observation of the primary disease and locoregional lymph nodes involvement(20,21). On T1-weighted images, the prostate appearance is homogeneous with isosignal, and the zonal anatomy and intraprostatic diseases are not demonstrated. These are observed on T2-weighted images, as the cancer presents itself as an area with signal hypointensity at peripheral zone, which is hyperintense(21). Technical advances for better signal detection by MRI antennas, have led to the development of endorectal coils(22). Magnetic resonance imaging endorectal coil presents > 97% accuracy in the localization of known prostate lesions; however, the method performance is poor in the detection of focal tumors with < 5 mm in diameter(23). Magnetic resonance spectroscopy of the prostate increases the diagnostic probability in cases of cancer, by adding metabolic data on the gland to the morphological information. The sensitivity of this method ranges from 68% to 95% and specificity, from 70% to 91%(21,24). Advantages of the utilization of this technique in the determination of prostate cancer include: accurate spectral localization of each small morphologically abnormal region; precise correlation between the spectral mapping and the high-resolution magnetic resonance imaging; evaluation of the abnormal metabolism extent; three-dimensional coverage of the entire gland(17). A variation is observed when MRI results and MRSI metabolic data are combined. Together, they result in 56-94% sensitivity and 70-98% specificity(10,24,25). In 2004, Yuen et al.(26) observed that MRI data in association with those of MRSI, presented 100% sensitivity and 70.3% specificity in the determination of suspicious areas. Most recently, in 2005, Prando et al.(27) observed that MRI combined with MRSI presented high sensitivity (84% to 100%) and low specificity (44% to 71%) in the identification of target areas. In the present study, alterations at MRI or at MRSI alone presented very low specificity. Thus, the findings should be considered when both peripheral zone hypointense signal at MRI and metabolic inversions at MRSI are present (58% accuracy). As regards sensitivity of MRI in association with MRSI in the detection of prostatic cancer (group A), the results of the present study are in agreement with previous studies. However, with respect to specificity, the results were below (47%) those described in the literature, and agreeing only with Prando et al.(27), including in what refers to the group in study. Therefore, information detected by MRSI with respect to the probable localization of prostate cancer may be useful in the programming of TRTRUS-guided biopsies, particularly in patients with PSA levels indicating cancer and with previous negative biopsies. It can also improve the stratification of patients in clinical screening, and also their monitoring, from a simple clinical follow up to a minimally aggressive treatment(17). The study protocol implementation underwent several phases. The first one occurred in 2004, with the installation of the Magneton Sonata MRI in the Department of Imaging Diagnosis, where commercially available Siemens MRI and spectroscopy pulse sequences were adapted to the working conditions. The second phase of prostate MRSI protocol set up corresponded to the elaboration of spectral analysis criteria. Based on these criteria, spectral data analysis was standardized, involving both qualitative and quantitative studies adapted according to recommendations in the literature(8,10-13,15-18,21,24-27,28) . Additionally, efforts were made to standardize studies reports and the TRTRUS-guided biopsies programming based on MRI and MRSI data. However, these phases were modified to better adapt to diagnosis requirements, not to mention the aspect of one being at the beginning of the learning curve. The valuation of certain metabolic inversions in the first patients contributed to the fact that accuracy results of the method fell short of the literature ones, approximately 58%. This difficulty in the diagnosis may be explained by the fact that MRSI as well as MRI results may be influenced by inflammatory processes (prostatitis), postbiopsy hemorrhages, and by several types of treatments such as hormone therapy, radiotherapy, cryotherapy among others(21,25,29). Concerning the mathematical relation, our study demonstrated that the utilization of creatine must be better evaluated, verifying to what extent its amplitude is being influenced by choline and spermidines. This was demonstrated by the higher number of false-positive results, with the mathematical relation (choline + creatine)/citrate.
CONCLUSION The implantation and standardization of magnetic resonance spectroscopy imaging allowed the acquisition of relevant data for the presumptive diagnosis of the presence of prostate cancer, combining the MR images with metabolic data from MRSI. Acknowledgement The authors wish to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), for the financial support.
REFERENCES 1. Silverberg E. Cancer statistics, 1985. CA Cancer J Clin. 1985;35:19-35. [ ] 2. American Cancer Society. Cancer facts & figures 2007. Atlanta: ACS; 2007. [ ] 3. Instituto Nacional de Câncer. Coordenação de Prevenção e Vigilância de Câncer. Estimativas 2008: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2007. [ ] 4. Carter HB, Sauvageot J, Walsh PC, et al. Prospective evaluation of men with stage T1C adenocarcinoma of the prostate. J Urol. 1997;157:2206-9. [ ] 5. Thompson I, Leach RJ, Pollock BH, et al. Prostate cancer and prostate-specific antigen: the more we know, the less we understand. J Natl Cancer Inst. 2003;95:1027-8. [ ] 6. Clements R. Contemporary ultrasound guided biopsy in the diagnosis of prostate cancer. Imaging. 2001;13:18-26. [ ] 7. Halpern EJ, Rosenberg M, Gomella LG. Prostate cancer: contrast-enhanced US for detection. Radiology. 2001;219:219-25. [ ] 8. Yu KK, Hricak H. Imaging prostate cancer. Radiol Clin North Am. 2000;38:59-85. [ ] 9. Punglia RS, D'Amico AV, Catalona WJ. Effect of verification bias on screening for prostate cancer by measurement of prostate-specific antigen. N Engl J Med. 2003;349:335-42. [ ] 10. Scheidler J, Hricak H, Vigneron DB, et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging - clinicopathologic study. Radiology. 1999;213:473-80. [ ] 11. Qayyum A, Coakley FV, Lu Y, et al. Organ-confined prostate cancer: effect of prior transrectal biopsy on endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenol. 2004;183:1079-83. [ ] 12. Yu KK, Scheidler J, Hricak, H, et al. Prostate cancer: prediction of extracapsular extension with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology. 1999;213:481-8. [ ] 13. Kurhanewicz J, Vigneron DB, Males RG, et al. The prostate: MR imaging and spectroscopy. Present and future. Radiol Clin North Am. 2000; 38:115-38. [ ] 14. Westphalen AC, Coakley FV, Qayyum A, et al. Peripheral zone prostate cancer: accuracy of different interpretative approaches with MR and MR spectroscopic imaging. Radiology. 2008;246: 177-84. [ ] 15. van der Graaf M, Schipper RG, Oosterhof GON, et al. Proton MR spectroscopy of prostatic tissue focused on the detection of spermine, a possible biomarker of malignant behavior in prostate cancer. Magn Reson Mat Biol Phys Med. 2000;10: 153-9. [ ] 16. Rajesh A, Coakley FV. MR imaging and MR spectroscopic imaging of prostate cancer. Magn Reson Imaging Clin N Am. 2004;12:557-79. [ ] 17. Kurhanewicz J, Vigneron DB, Hricak H, et al. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24-0.7 cm3) spatial resolution. Radiology. 1996;198: 795-805. [ ] 18. Thomas MA, Narayan P, Kurhanewicz J, et al. 1H MR spectroscopy of normal and malignant human prostates in vivo. J Magn Reson. 1990;87:610-9. [ ] 19. McNeal JE. The prostate gland: morphology and pathobiology. Monogr Urol. 1983;4:5-13. [ ] 20. Outwater EK, Petersen RO, Siegelman ES, et al. Prostate carcinoma: assessment of diagnostic criteria for capsular penetration on endorectal coil MR images. Radiology. 1994;193:333-9. [ ] 21. Shukla-Dave A, Hricak H, Eberhardt SC, et al. Chronic prostatitis: MR imaging and 1H MR spectroscopic imaging findings - initial observations. Radiology. 2004;231:717-24. [ ] 22. Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific-antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868-78. [ ] 23. Beyersdorff D, Taupitz M, Winkelmann B, et al. Patients with a history of elevated prostate-specific antigen levels and negative transrectal US-guided quadrant or sextant biopsy results: value of MR imaging. Radiology. 2002;224:701-6. [ ] 24. Ackerstaff E, Pflug BR, Nelson JB, et al. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer Res. 2001;61:3599-603. [ ] 25. Zakian KL, Eberhardt S, Hricak H, et al. Transition zone prostate cancer: metabolic characteristics at 1H MR spectroscopic imaging - initial results. Radiology. 2003;229:241-7. [ ] 26. Yuen JS, Thng CH, Tan PH, et al. Endorectal magnetic resonance imaging and spectroscopy for the detection of tumor foci in men with prior negative transrectal ultrasound prostate biopsy. J Urol. 2004;171:1482-6. [ ] 27. Prando A, Kurhanewicz J, Borges AP, et al. Prostatic biopsy directed with endorectal MR spectroscopic imaging findings in patients with elevated prostate specific antigen levels and prior negative biopsy findings: early experience. Radiology. 2005;236:903-10. [ ] 28. Amsellem-Ouazana D, Younes P, Conquy S, et al. Negative prostatic biopsies in patients with a high risk of prostate cancer: is the combination of endorectal MRI and magnetic resonance spectroscopy imaging (MRSI) a useful tool? A preliminary study. Eur Urol. 2005;47:582-6. [ ] 29. Ikonen S, Kivisaari L, Tervahartiala P, et al. Prostatic MR imaging. Accuracy in differentiating cancer from other prostatic disorders. Acta Radiol. 2001;42:348-54. [ ] Received May 28, 2008. * Study developed at the Department of Imaging Diagnosis - Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554