Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 1 - Jan. /Feb. of 2009
Vol. 42 nº 1 - Jan. /Feb. of 2009
|
WHICH IS YOUR DIAGNOSIS?
|
|
Which is your diagnosis? |
|
|
Autho(rs): Marcelo Souto Nacif, Bianca de Cássia Cavalieri, Amarino Carvalho de Oliveira Junior, Claudio Assunção, Tiago Souto Nacif, Evandro Tinoco Mesquita |
|
|
IFellow PhD degree (Cardiac MRI) in Radiology, Universidade Federal do Rio de Janeiro (UFRJ), MD, Unit of Radiology and Imaging Diagnosis - Hospital Pró-Cardíaco, Rio de Janeiro, RJ, Brazil
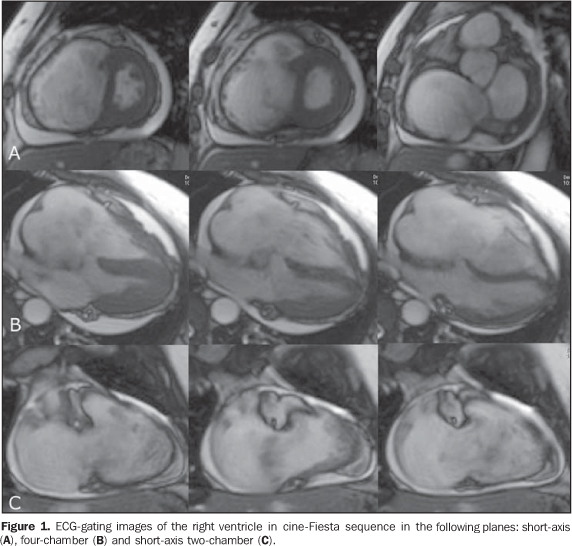
A female, 55-year-old patient, weighting 57 kg, with 1.55 m in height, cardiac frequency of 95 bpm, presenting with episodes of palpitation and dyspnea associated with signs of right heart failure. Cardiac auscultation demonstrated protodiastolic murmur suggesting obstruction of the tricuspid flow. The other valves were normal. Plain chest radiography (lateral and postero-anterior views) demonstrated enlarged right heart cavities. Echocardio-graphy identified a mass in the region of the tricuspid valve and significant right ventricular and atrial enlargement. The patient was referred to the Unit of Radiology and Imaging Diagnosis of Hospital Pró-Car-díaco for evaluation of the cardiac mass. Images description Figure 1. ECG-gating images of the right ventricle in cine-Fiesta sequence in the following planes: short-axis (A), four-chamber (B) and short-axis two-chamber (C). A bulging mass measuring 7.5 × 5.0 × 4.6 cm is observed, with heterogeneous and predominantly high signal intensity on T2-weighted sequences, with a pedicle inserted into the right atrium, above de venous sinus, insinuating through the tricuspid valve into the ventricle during the ventricular diastole, bulging the interventricular septum and the free right ventricular wall. Note the enlarged right heart cavities. Hyperkinetic left ventricle with preserved diameter, global and segmental functions, with estimated 82% ejection fraction. Subtle/mild pericardial effusion. Enlargement of the inferior vena cava and signs of restricted blood flow in the right heart cavities.
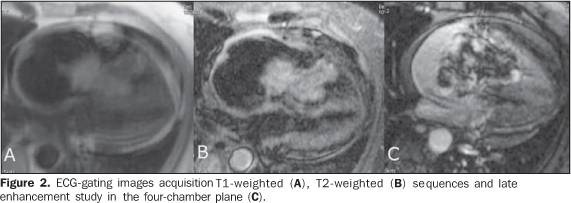
Figure 2. ECG-gating images acquisition T1-weighted (A), T2-weighted (B) sequences and delayed enhancement study in the four-chamber plane (C). Note that the mass presents heterogeneous and intermediate signal intensity on the T1-weighted sequence, with high signal intensity on the T2-weighted sequence in association with a significant gadolinium enhancement in the central portion and adjacent to the mass pedicle, with low signal intensity in the lesion periphery.
Diagnosis: Right atrial myxoma.
COMMENTS Myxomas represent the most frequent type of primary benign cardiac tumors in adult individuals accounting for approximately 50% of primary cardiac neoplasms, with only 17% being found in children. Generally, myxomas are solitary, occurring predominantly in women with mean age of 51, although this type of tumor may be found at any age range(1,2). It is important to note that about 75-80% of these tumors occur in the left atrium, 10-20% in the right atrium, and 5-10% in both atria. Other structures, such as aorta, pulmonary artery, ventricles, heart valves, or even other organs such as lungs, and bones may be affected(1,2). Despite their benign histological nature, myxomas may present unfavorable progression, resulting in complications and even sudden death, considering the possible occurrence of embolic pulmonary or systemic phenomena resulting from the tumor fragmentation or even valvar obstruction depending on the tumor localization and dimensions(1-3). Signs and symptoms are not typical or specific and may mimic from stenosis and/or mitral failure to Budd-Chiari syndrome, depending on the tumor site and extent. The most frequent symptoms include dyspnea, paroxysmal dyspnea, syncope, palpitation, chest pain, embolic event, fever and weight loss. Most frequently the following sings are observed: systolic or diastolic murmur in the affected valve, B1 hyperphonetic sound and tumor noise, also depending on the tumor site. On the other hand, laboratory tests results are concerned with increased hemosedimentation rate, thrombocytopenia, polycythemia, anemia, increased globulin and C-reactive protein levels, leukocytosis. Electrocardiogram and chest teleradiography may show either normal results or cardiac involvement, but do not provide disease-specific data. Electrocardiography can detect the presence of arrhythmia, heart chambers overload, changes in ventricular repolarization and conductive disorders(2,4). The presence of these manifestations is observed in only 33.3% of patients, generally requiring the differential diagnosis with other conditions, particularly valvar disease and rheumatic fever(4). Generally, right atrial myxomas originate in the atrial cavity, the most common site of attachment being the border of the oval fossa (more than 60% of cases) and less frequently, the atrial wall or the lower interatrial septum, as well as the atrioventricular valve(2,3). Also, it is important to mention the occurrence of familial myxomas, besides syndromic presentations (Carney's complex) including alterations such as lentigines and/or nevi, nodular adrenocortical disease, Cushing's syndrome, myxoid fribroadenoma, hypophyseal and testicular tumors. NAME syndrome corresponds to the acronym for nevi, atrial myxoma, myxoid neurofibroma and ephelides, and LAMB syndrome, to lentigines, atrial myxomas, mucocutaneous myxomas and blue nevi. Syndromic presentations and familial myxomas are more likely to be multiple and at risk of recurrence, occurring at lower age ranges, around the second decade of life(3). The role of cardiac magnetic resonance imaging (cardiac MRI) Cardiac MRI with multiplanar sequences in cine-MRI can demonstrate the macroscopic feature of the tumor, quantifying the volume (Figure 3), motility and, in some cases, prolapse through the atrioventricular valve, besides characterizing the signal intensity and the actual attachment site on the endocardial surface(2,5). The T1-weighted sequences demonstrate the signal isointensity relative to the cardiac muscle(2,5). It is important to note that myxomatous components present low signal intensity on T1-, and high signal intensity on T2-weighted sequences, and that calcifications will present low signal intensity on T1- and T2-weighted sequences(2,5). As regards hemorrhagic foci, the signal intensity will depend on the time of bleeding as follows: intermediate signal intensity on T1- and high signal intensity on T2-weighted sequences in hyperacute hemorrhages (oxyhemoglobulin - up to 1 hour), intermediate signal intensity on T1- and low signal intensity on T2-weighted sequences in acute hemorrhages (deoxyhe-moglobulin - between 1 and 24 hours), high signal intensity on T1- and low signal intensity on T2-weighted sequences in early subacute hemorrhages (intracellular methemoglobin - between 24 hours and 1 week), high signal intensity on T1- and T2-weighted sequences in late subacute hemorrhage (extracellular methemoglobin - between 24 hours and 1 week), and low signal intensity on T1- and T2-weighted sequences in chronic hemorrhages (hemosiderin - more than 1 month)(2,5). Heterogeneous gadolinium enhancement is typical, resulting from the presence of inflammation or growing tumor-like tissue associated with the presence of unenhanced areas corresponding to necrosis, cysts or attached thrombi(2,5). The surgical procedure Non-invasive methods, especially MRI, have brought a large contribution to the diagnosis of intracardiac myxomas, considering that, besides confirming the diagnosis, these methods allow the planning of appropriate surgical techniques(1,3). Excision of atrial myxomas can be performed by means of transseptal approach or atriotomy. A careful manipulation of cardiac structures as well as of the tumor during excision reduces the possibility of tumor fragmentation and occurrence of embolic phenomena. A wider resection of the stalk attachment to the endocardium must be performed to prevent any residual tissue to cause tumor recurrence. Cauterization of resection borders is frequently performed, and pericardial or Teflon patches may be utilized for reconstruction as necessary. Surgical resection is performed with low mortality and complication rates. Tumor recurrence rates range between 1% and 5%(1,3,4). In the present case (Figure 4), the tumor was approached by means of atriotomy, requiring valvar reconstruction because of the tumor size compatible with the values described by MRI. There was no technical difficulty or complication during the surgery. The patient is currently under follow-up. Pathological findings Myxomas histogenesis is uncertain, but there are evidences that the tumor originates from subendocardial nests of primitive mesenchymal cells differentiated into several cell types, including endothelial and lipidic cells. Cytogenetic analysis demonstrates clonal chromosomal abnormalities on which this concept is based(3). Generally, myxomas are pediculated and may present macroscopic variants. This tumor may present with a stiff, myxoid, gelatinous or friable surface; lobulated or irregular in shape. Additionally calcifications, hemorrhagic foci and polypoid formations. The tumor volume depends on the disease development phase(1,3-5). In the present case, the histopathological analysis has evidenced the presence of star-shaped cells, some of them arranged in cords intermingled with the myxoid matrix, confirming the diagnosis of myxoma. Final considerations Cardiac MRI is a widely capable and essential non-invasive diagnostic method, being useful in the surgical planning, considering the relevance of an early diagnosis and appropriate surgical resection to improve the prognosis and preventing complications and mortality in cases of atrial myxomas.
REFERENCES 1. Miralles A, Bracamonte L, Soncul H, et al. Cardiac tumors: clinical experience and surgical results in 74 patients. Ann Thorac Surg. 1991;52: 886-95. [ ] 2. Khin MM, Kwong RY. Cardiac and pericardial tumors. In: Kwong RY, editor. Cardiovascular magnetic resonance imaging. 1st ed. New Jersey: Humana Press; 2008; p. 429-65. [ ] 3. Motta AAR, Colen Filho E, Colen EA, et al. Mixoma do átrio esquerdo: relato de 3 casos. Rev Bras Cir Cardiovasc. 1997;12:377-83. [ ] 4. Sabatine MS, Colucci WS, Schoen FJ. Tumores primários do coração. In: Zipes DP, Libby P, Bonow RO, et al., editors. Braunwald - Tratado de doenças cardiovasculares. 7ª ed. Rio de Janeiro: Elsevier; 2006. p. 1741-55. [ ] 5. Abbara S, Walker TG, Ng P, et al. Diagnostic imaging - cardiovascular. Manitoba: Amirsys; 2008. [ ] Study developed in the Unit of Radiology and Imaging Diagnosis at Hospital Pró-Cardíaco and Department of Radiology - Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554