Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 55 nº 5 - Sep. / Oct. of 2022
Vol. 55 nº 5 - Sep. / Oct. of 2022
|
ORIGINAL ARTICLE
|
|
18F-FDG-PET/CT-measured parameters as potential predictors of residual disease after neoadjuvant chemoradiotherapy in patients with esophageal carcinoma |
|
|
Autho(rs): Francisco Tustumi1,a; David Gutiérrez Albenda2,b; Rubens Antonio Aissar Sallum1,c; Sergio Carlos Nahas1,d; Ulysses Ribeiro Junior1,e; Carlos Alberto Buchpiguel2,f; Ivan Cecconello1,g; Paulo Schiavom Duarte2,h |
|
|
Keywords: Esophageal neoplasms; Neoadjuvant therapy; Positron-emission tomography; Nuclear medicine. |
|
|
Abstract: INTRODUCTION
In locally advanced resectable esophageal cancer, neoadjuvant chemoradiotherapy (nCRT) is currently the standard of care(1,2). After nCRT, pathological regression is the main prognostic indicator for esophageal cancer(3). Currently, patients with esophageal cancer undergo surgical resection of the primary lesion after nCRT regardless of the pathological status of the lesion after the therapy. However, for patients with other types of cancer, such as rectal cancer(4), a watchful waiting approach is often adopted if clinical staging suggests a pathological complete response (pCR) after nCRT. Therefore, if a diagnostic imaging method could accurately identify a pCR after nCRT in patients with esophageal cancer, it would also be possible to identify patients who are the best candidates for a watchful waiting approach, somewhat similar to what is done in cases of rectal cancer(4), thus avoiding the postoperative complications and high mortality associated with esophagectomy. Functional imaging, such as 18F-fluorodeoxyglucose positron-emission tomography/computed tomography (18F- FDG-PET/CT), evaluates metabolic activity and may improve patient selection for further treatment(5). There have been studies demonstrating the prognostic value of metabolic tumor volume (MTV) and total lesion glycolysis (TLG) for esophageal cancer treated with trimodal therapy(6–13). However, the use of the 18F-FDG-PET/CT to assess the presence of residual disease (RD) after nCRT in patients with esophageal cancer has not been widely studied. In lung and rectal cancer, serial 18F-FDG-PET/CT after neoadjuvant therapy can identify predictors of a pathological response(14,15). However, studies using 18F-FDG-PET/CT after nCRT in patients with esophageal cancer have produced heterogeneous results. Heneghan et al.(16) evaluated the accuracy of the post-nCRT maximum standardized uptake value (SUVmax) in detecting a pCR and found it to have a sensitivity of only 56%. McLoughlin et al.(17) showed that 18F-FDG-PET/CT had a sensitivity of 62% and specificity of 44% for the detection of a pCR after nCRT. Gabrielson et al.(18) reported significantly greater SUV changes in nCRT responders than in nCRT nonresponders. However, the authors found no significant difference in SUV between the patients with a pCR and those with a subtotal response. In addition, Elliott et al.(19) evaluated esophageal adenocarcinoma only and showed that 18F-FDG-PET/CT had poor discriminatory value for clinical application. However, those studies did not evaluate the volumetric parameters determined by 18F-FDG-PET/CT and used distinct nCRT regimens. Therefore, our study aims to evaluate the potential of the SUVmax, mean SUV (SUVmean), MTV, and TLG measured at the site of primary esophageal tumor on 18F-FDG-PET/CT performed before and after nCRT with a platinum- and taxane-based regimen, as well as the change in those values between the two time points and their association with RD. MATERIALS AND METHODS Patients and study design This was a retrospective cross-sectional study of patients with esophageal carcinoma, in which we attempted to determine whether the SUVmax, SUVmean, and volumetric parameters (MTV and TLG) obtained by 18F-FDG-PET/CT, before and after nCRT, are associated with the pathological response. We recruited patients from among those under treatment at a single institute between 2009 and 2019. We included 39 patients who had completed a (platinum- and taxane-based) nCRT regimen, followed by esophagectomy with curative intent, and had undergone 18F-FDG-PET/CT at least twice: before nCRT; and between the end of nCRT and the esophagectomy. The nCRT included the administration of carboplatin or cisplatin concurrent with radiation (41.4, 45.0, or 50.4 cGy). The patients were staged with endoscopy, CT, and 18F-FDG-PET/CT, after which they were classified according to the 8th edition of the Union for International Cancer Control staging system(20). The local research ethics committee approved the study (Reference no. 1492/19). An experienced pathologist, blinded to the 18F-FDG-PET/CT findings, evaluated the surgical specimens. A pCR to nCRT was defined as no residual malignant cells detected by hematoxylin and eosin staining in the surgical specimen. We defined RD as the presence of any cancer cells (single cells or cell clusters). 18F-FDG-PET/CT acquisition and imaging analyses A whole-body PET/CT system with time-of-flight capability (Discovery 690; GE Healthcare, Waukesha, WI, USA) was used for the 18F-FDG-PET/CT acquisition. The patients were instructed to fast for at least six hours and were required to have a blood glucose level ≤ 180 mg/dL before injection of the 18FDG (≤ 3.7 MBq/kg of body weight). Image acquisition was initiated approximately 60 min after injection of the radiotracer, and images were acquired from the mid-skull to the mid-thigh. The metabolic activity at the primary tumor site, before and after nCRT, was recorded by using the following parameters: SUVmax, SUVmean, TLG, and MTV. Those values were calculated by a single nuclear medicine physician using a radiology workstation (AW VolumeShare 5; GE Healthcare). The SUV thresholds used in order to define the boundaries of the lesions were established by visual analysis. The total volume of interest that circumscribed the metabolic area was calculated automatically by the dedicated software. Statistical analysis Receiver operating characteristic (ROC) curves were used in order to establish the sensitivity and specificity of the distinct operating points of the four parameters measured before and after nCRT, as well as the differences between the pre- and post-nCRT values, thus allowing the post-nCRT status of the primary tumor site to be classified as a pCR or RD. The result of the histopathological analysis of the surgical specimen was used as the gold standard. The areas under the curve (AUCs) and the corresponding 95% confidence intervals (95% CIs) were used in order to evaluate the accuracy of the four parameters in that classification. Finally, we estimated the sensitivity and specificity of those parameters for the identification of RD when no cancer cells were detected through visual inspection of the primary tumor site after nCRT. Any residual uptake above the background level at the primary tumor site was used as the threshold. Descriptive statistics—including means and standard deviations; medians and ranges; and absolute and relative frequencies—are presented for some variables. We also present the sensitivity and specificity of the best operation point to classify the patients as achieving a pCR or having RD. Statistical analyses were performed with the Stata software package, version 16.1 (StataCorp, College Station, TX, USA). The level of statistical significance was set at p < 0.05. RESULTS Patient baseline characteristics Our analysis included 39 patients who underwent 18F-FDG-PET/CT before nCRT with a platinum- and taxane-based regimen and between the nCRT and the esophagectomy. The mean time from the pre-treatment 18F-FDG-PET/CT to the beginning of the nCRT was 12 ± 6 weeks. The mean time from the last cycle of the nCRT to the post-treatment 18F-FDG-PET/CT was 9 ± 4 weeks, and the mean time from the end of nCRT to surgery was 16 ± 6 weeks. The two chemotherapy regimens adopted were carboplatin plus paclitaxel (in 54% of the patients) and cisplatin plus paclitaxel (in 46%). The radiation doses used were 41.4 cGy (in 54% of the patients), 45.0 cGy (in 28%), and 50.4 cGy (in 18%). The 90-day mortality rate was 15.4% (Table 1). 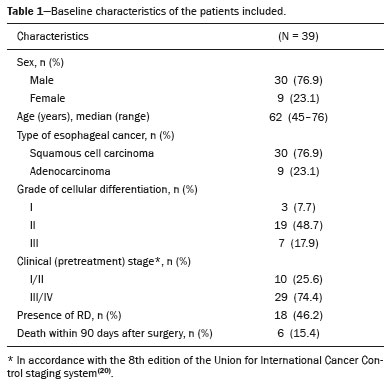 Pathological response The ROC curve analyses of the variables determined by 18F-FDG-PET/CT, in terms of their ability to predict RD, are shown in Table 2, as well as in Figures 1 and 2. The pre-treatment values for the 18F-FDG-PET/CT parameters were not found to be predictors of RD. For the post-treatment 18F-FDG-PET/CT parameters, the AUCs were similar among all four of the variables related to the primary tumor and those AUCs were statistically significant (Figure 1). After nCRT, the AUC was 0.7169 (95% CI: 0.5541–0.8797) for the SUVmax, 0.7169 (95% CI: 0.5537–0.8801) for the MTV, 0.7196 (95% CI: 0.5544–0.8848) for the SUVmean, and 0.709 (95% CI: 0.5449–0.8731) for the TLG. For the difference between the pre- and post-treatment values of the four 18F-FDG-PET/CT parameters, only the SUV parameters (SUVmax and SUVmean) showed significant ability to detect RD (Figure 2). Table 2 summarizes those findings. 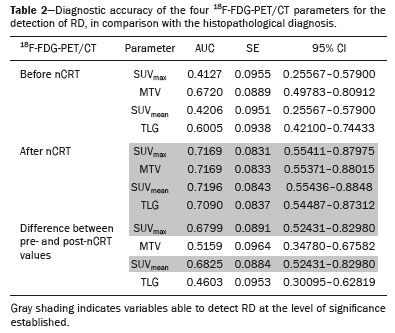 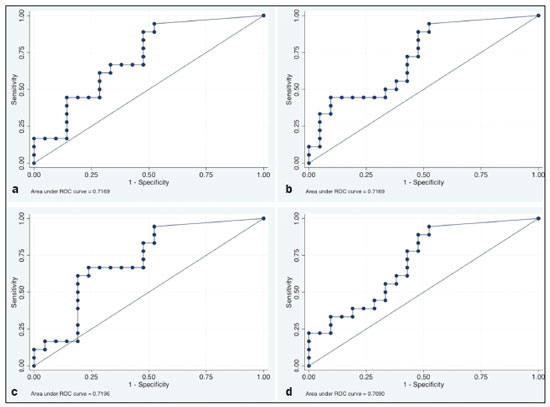 Figure 1. ROC curves of the accuracy of the four 18F-FDG-PET/CT parameters after nCRT for the detection of RD, in comparison with the histopathological diagnosis. a: SUVmax. b: MTV. c: SUVmean. d: TLG. 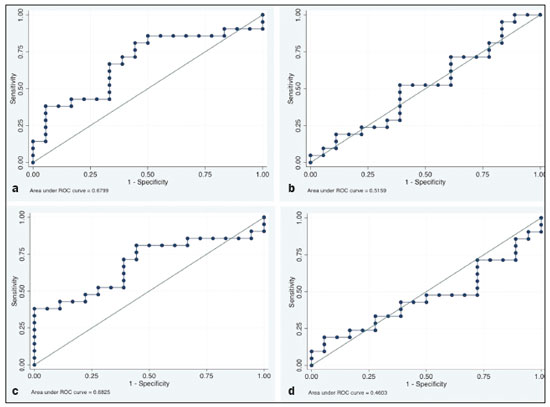 Figure 2. ROC curves of the accuracy of the difference between the pre- and post-treatment values of the four 18F-FDG-PET/CT parameters for the detection of RD, in comparison with the histopathological diagnosis. a: SUVmax. b: MTV. c: SUVmean. d: TLG. Given that the main utility of 18F-FDG-PET/CT in esophageal cancer is to detect all patients with RD, which improves the selection of surgical candidates, the sensitivity of the modality is more important than is its specificity. Therefore, if the SUV or volumetric parameters of the primary tumor on 18F-FDG-PET/CT after nCRT were not “zero” (i.e., the nuclear medicine physician visually detected a region of uptake above the background level at the primary tumor site), the sensitivity for predicting RD was high (94.4%; 95% CI: 72.7–99.9%), although the specificity was intermediate (47.6%; 95% CI: 25.7–70.2%). Figure 3 depicts a patient with residual uptake at the primary tumor site after nCRT. Figure 4 depicts another patient, in whom there was no visually detectable uptake after nCRT. Using any residual uptake above the background level as the threshold, we identified 17 of the 18 patients with RD and 10 of the 21 patients with a pCR (Table 3). 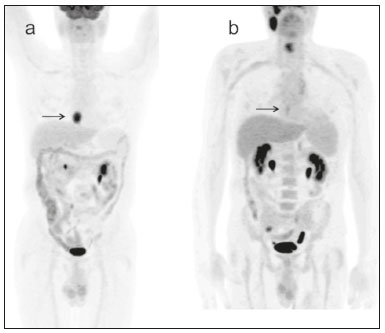 Figure 3. a: Maximum intensity projection (MIP) image of the 18F-FDG-PET/CT scan acquired before nCRT, showing intense uptake at the primary tumor site in the esophagus (arrow). b: MIP of the 18F-FDG-PET/CT scan acquired after nCRT, showing faint uptake at the primary tumor site (arrow). The patient presented RD. There was also nonspecific diffuse uptake in the right masseter and lateral pterygoid muscles, which could be explained by muscle contraction, given that there were no evident anatomical alterations. 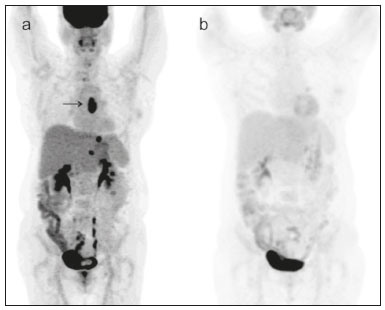 Figure 4. a: MIP image of the 18F-FDG-PET/CT scan acquired before nCRT, showing intense uptake at the primary tumor site in the esophagus (arrow). b: MIP of the 18F-FDG-PET/CT scan acquired after nCRT, showing no detectable uptake at the primary tumor site. The patient presented a pCR. 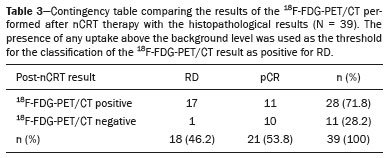 DISCUSSION In this study of patients with esophageal cancer, we have shown that the 18F-FDG-PET/CT parameters obtained for the primary tumor site after nCRT has the potential to predict RD. In this context, the presence of any visually detectable uptake above the background level at the site of the tumor seems to be the best predictor of RD. Our results suggest that some patients without visually detectable uptake on 18F-FDG-PET/CT after nCRT would benefit from a watchful waiting approach, somewhat similar to that applied in cases of rectal cancer(4), which avoids the risk of postoperative complications and mortality associated with esophagectomy. Such patients accounted for approximately 28% of our sample. If a watchful waiting approach had been adopted in those cases, the overall mortality rate could have been lower. Our study has some limitations. First, it was a retrospective, single-center study with small sample size. In addition, although our results provide evidence to support the use of 18F-FDG-PET/CT to predict RD after nCRT, the criterion used for 18F-FDG-PET/CT classification as positive (any uptake above the background level) is not perfect and failed to detect RD in one of the 18 patients with RD in our sample. Therefore, the method should be used with extreme caution for surgical decision-making, and every choice should be shared with the patient and their family. As a general rule, patients should still be referred for resection. Nevertheless, 18F-FDG-PET/CT could be useful in some cases in which surgery is indicated. For example, in patients who deteriorate in the setting of neoadjuvant therapy, who are at higher risk of complications of esophagectomy, and in whom the 18F-FDG-PET/CT variables favor the attainment of a pCR, the medical team could offer, in consultation with the patient and their family, the option of adopting a watchful waiting approach. Despite the fact that the SUV and volumetric parameters are known to be associated with the long-term prognosis in esophageal cancer(6–13,21), there are divergent results in the literature regarding the utility of 18F-FDG-PET/CT in predicting the pathological response to nCRT(11,22,23). Most of the relevant studies have used a variety of neoadjuvant regimens, have not assessed th 18F-FDG-PET/CT parameters after nCRT, and have not taken a standardized approach to data analysis. Arnett et al.(22) showed that the 18F-FDG-PET/CT parameters (SUVmax, SUVmax normalized to liver uptake, and SUVmax normalized to blood pool uptake) measured pre- and post-nCRT were not significantly associated with a pCR in a sample of 193 patients, most of whom had adenocarcinoma. However, the authors did not use ROC curves to define a metabolic threshold to separate patients with a pCR from those with RD. In addition, most of the patients in our sample had squamous cell carcinoma, which is known to have a higher probability of a pathological response after chemoradiotherapy, as demonstrated elsewhere(24). That could explain the superiority of our results. In a recent study, Choi et al.(25), analyzing a cohort of patients undergoing trimodal therapy, showed that pre- and post-treatment volumetric parameters from 18F-FDG-PET/CT are independent variables associated with a pCR. However, those authors evaluated 18F-FDG-PET/CT parameters only as prognostic variables and did not propose the use of 18F-FDG-PET/CT as a diagnostic tool for detecting RD. Currently, clinicians lack a reliable tool to facilitate the decision between surgery and a watchful waiting approach after nCRT. Our study provides evidence that 18F-FDG-PET/CT could be a useful tool for the detection of RD and has high sensitivity when any residual uptake above the background level at the primary tumor site is used as a threshold. It should be borne in mind that an inflammatory reaction due to radiation exposure may partially explain the low specificity of 18F-FDG uptake in the definition of RD. Previous studies have suggested that an inflammatory response could have a confounding effect on the 18F-FDG-PET/CT variables after neoadjuvant therapy(26,27). Therefore, the interval between the end of the nCRT and the post-treatment 18F-FDG-PET/CT may be a key factor that could explain some of the variability of the results among the studies and could contribute to the limited specificity. In the present study, the mean time from the last cycle of the nCRT to the post-treatment 18F-FDG-PET/CT was relatively short (nine weeks). It is possible that a longer interval would have increased the specificity. Therefore, future studies should attempt to determine whether a longer interval between the last nCRT cycle and 18F-FDG-PET/CT could improve the accuracy of the examination in the identification of RD. It is noteworthy that, in our study, any residual uptake above the background level at the primary tumor site was found to be the best threshold to classify the 18F-FDG-PET/CT study as positive or negative for RD. Therefore, the quantitative and semiquantitative parameters (SUVmax, SUVmean, MTV, and TLG) do not appear to be fundamental for the purpose of defining the pathological response, although that should be better analyzed in future studies. Combining the 18F-FDG-PET/CT data with those obtained by other diagnostic methods or with clinical and demographic data could increase the accuracy for the identification of a pCR. In a study of esophageal squamous cell cancer conducted by Molena et al.(28), a ≥ 70% reduction in the SUVmax combined with a normal appearance on endoscopy and a lack of RD on biopsy was found to increase the chance of achieving a pCR. Zhang et al.(29) analyzed a model to predict a pCR that combines 18F-FDG-PET/CT, clinical data, and demographic features. They found that the model accurately predicted a pCR. Therefore, future analyses of our data are necessary to determine whether combining the results of the 18F-FDG-PET/CT study with clinical and demographic parameters, as well as with the results of other diagnostic tests, could further improve the accuracy for the detection of pCR. The results of the present study do not provide a definitive answer for clinicians who manage esophageal cancer and should be interpreted in the context of the aforementioned limitations. Therefore, larger, controlled prospective studies are warranted in order to determine the true accuracy of 18F-FDG-PET/CT for the detection of RD. CONCLUSION The use of 18F-FDG-PET/CT after neoadjuvant therapy in patients with esophageal carcinoma has the potential to predict the pathological response. The parameters measured by 18F-FDG-PET/CT also facilitate the selection of patients who are eligible for a watchful waiting approach. REFERENCES 1. van Hagen P, Hulshof MCCN, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. 2. Mota FC, Cecconello I, Takeda FR, et al. Neoadjuvant therapy or upfront surgery? A systematic review and meta-analysis of T2N0 esophageal cancer treatment options. Int J Surg. 2018;54(Pt A): 176–81. 3. Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–7. 4. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7. 5. Odawara S, Kitajima K, Katsuura T, et al. Tumor response to neoadjuvant chemotherapy in patients with esophageal cancer assessed with CT and FDG-PET/CT – RECIST 1.1 vs. PERCIST 1.0. Eur J Radiol. 2018;101:65–71. 6. Bütof R, Hofheinz F, Zöphel K, et al. Prognostic value of pretherapeutic tumor-to-blood standardized uptake ratio in patients with esophageal carcinoma. J Nucl Med. 2015;56:1150–6. 7. Elimova E, Wang X, Etchebehere E, et al. 18-fluorodeoxy-glucose positron emission computed tomography as predictive of response after chemoradiation in oesophageal cancer patients. Eur J Cancer. 2015;51:2545–52. 8. Hatt M, Visvikis D, Albarghach NM, et al. Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011;38:1191–202. 9. Hofheinz F, Li Y, Steffen IG, et al. Confirmation of the prognostic value of pretherapeutic tumor SUR and MTV in patients with esophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2019;46:1485–94. 10. Hong JH, Kim HH, Han EJ, et al. Total lesion glycolysis using 18F-FDG PET/CT as a prognostic factor for locally advanced esophageal cancer. J Korean Med Sci. 2016;31:39–46. 11. Jayachandran P, Pai RK, Quon A, et al. Postchemoradiotherapy positron emission tomography predicts pathologic response and survival in patients with esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:471–7. 12. Lemarignier C, Di Fiore F, Marre C, et al. Pretreatment metabolic tumour volume is predictive of disease-free survival and overall survival in patients with oesophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2014;41:2008–16. 13. Li Y, Lin Q, Luo Z, et al. Value of sequential 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) in prediction of the overall survival of esophageal cancer patients treated with chemoradiotherapy. Int J Clin Exp Med. 2015;8:10947–55. 14. Cerfolio RJ, Bryant AS, Winokur TS, et al. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg. 2004; 78:1903–9. 15. Maffione AM, Marzola MC, Capirci C, et al. Value of (18)F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am J Roentgenol. 2015; 204:1261–8. 16. Heneghan HM, Donohoe C, Elliot J, et al. Can CT-PET and endoscopic assessment post-neoadjuvant chemoradiotherapy predict residual disease in esophageal cancer? Ann Surg. 2016;264:831–8. 17. McLoughlin JM, Melis M, Siegel EM, et al. Are patients with esophageal cancer who become PET negative after neoadjuvant chemoradiation free of cancer? J Am Coll Surg. 2008;206:879–86. 18. Gabrielson S, Sanchez-Crespo A, Klevebro F, et al. 18F FDG-PET/CT evaluation of histological response after neoadjuvant treatment in patients with cancer of the esophagus or gastroesophageal junction. Acta Radiol. 2019;60:578–85. 19. Elliott JA, O’Farrell NJ, King S, et al. Value of CT-PET after neoadjuvant chemoradiation in the prediction of histological tumour regression, nodal status and survival in oesophageal adenocarcinoma. J Brit Surg. 2014;101:1702–11. 20. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119–30. 21. Tustumi F, Duarte PS, Albenda DG, et al. Prognostic value of 18F-fluorodeoxyglucose PET/computed tomography metabolic parameters measured in the primary tumor and suspicious lymph nodes before neoadjuvant therapy in patients with esophageal carcinoma. Nucl Med Commun. 2021;42:437–43. 22. Arnett ALH, Merrell KW, Macintosh EM, et al. Utility of 18F-FDG PET for predicting histopathologic response in esophageal carcinoma following chemoradiation. J Thorac Oncol. 2017;12:121–8. 23. Wieder HA, Brücher BLDM, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–8. 24. Takeda FR, Tustumi F, Obregon CA, et al. Prognostic value of tumor regression grade based on Ryan score in squamous cell carcinoma and adenocarcinoma of esophagus. Ann Surg Oncol. 2020;27:1241–7. 25. Choi Y, Choi JY, Hong TH, et al. Trimodality therapy for locally advanced esophageal squamous cell carcinoma: the role of volume-based PET/CT in patient management and prognostication. Eur J Nucl Med Mol Imaging. 2022;49:751–62. 26. Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4692–8. 27. Schmidt M, Bollschweiler E, Dietlein M, et al. Mean and maximum standardized uptake values in [18F]FDG-PET for assessment of histopathological response in oesophageal squamous cell carcinoma or adenocarcinoma after radiochemotherapy. Eur J Nucl Med Mol Imaging. 2009;36:735–44. 28. Molena D, Sun HH, Badr AS, et al. Clinical tools do not predict pathological complete response in patients with esophageal squamous cell cancer treated with definitive chemoradiotherapy. Dis Esophagus. 2014;27:355–9. 29. Zhang H, Tan S, Chen W, et al. Modeling pathologic response of esophageal cancer to chemoradiation therapy using spatial-temporal 18F-FDG PET features, clinical parameters, and demographics. Int J Radiat Oncol Biol Phys. 2014;88:195–203. 1. Department of Gastroenterology, Digestive Surgery Division, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil 2. Department of Radiology and Oncology, Nuclear Medicine Division, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil a. https://orcid.org/0000-0001-6695-0496 b. https://orcid.org/0000-0002-5879-5382 c. https://orcid.org/0000-0003-1823-0042 d. https://orcid.org/0000-0002-2268-4146 e. https://orcid.org/0000-0003-1711-7347 f. https://orcid.org/0000-0003-0956-2790 g. https://orcid.org/0000-0002-3535-4170 h. https://orcid.org/0000-0003-4517-4410 Correspondence: Dr. Francisco Tustumi Hospital das Clínicas – FMUSP Avenida Doutor Eneas de Carvalho Aguiar, 255, Cerqueira César São Paulo, SP, Brazil, 05403-000 Email: franciscotustumi@gmail.com Received 24 August 2021 Accepted after revision 27 October 2021 Publication date: 24/02/2022 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554