Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 54 nº 5 - Sep. / Oct. of 2021
Vol. 54 nº 5 - Sep. / Oct. of 2021
|
ORIGINAL ARTICLES
|
|
The ratio between the whole-body and primary tumor burden, measured on 18F-FDG PET/CT studies, as a prognostic indicator in advanced non-small cell lung cancer |
|
|
Autho(rs): Felipe Renê Alves Oliveiraa; Allan de Oliveira Santosb; Mariana da Cunha Lopes de Limac; Ivan Felizardo Contrera Torod; Thiago Ferreira de Souzae; Bárbara Juarez Amorimf; Aristoteles Souza Barbeirog; Elba Etchebehereh |
|
|
Keywords: Fluorodeoxyglucose F18; Positron-emission tomography/methods; Tomography, X-ray computed/methods; Carcinoma, non-small-cell lung/diagnosis; Tumor burden; Carcinoma, non-small-cell lung/mortality. |
|
|
Abstract: INTRODUCTION
Lung cancer is the leading cause of death worldwide(1,2). Non-small-cell lung cancer (NSCLC) accounts for 80% of all cases of lung cancer, and more than half of all patients with NSCLC have metastatic disease at the time of diagnosis, the 5-year survival rate for all stages combined being 18%(2). For advanced NSCLC, the primary treatment modalities are chemotherapy and chemoradiotherapy(1,2). Overall survival (OS) is low in NSCLC even in the earlier tumor-node-metastasis (TNM) stages, decreasing progressively as the TNM stage increases—from 50% in stage IA to 2% in stage IV(2). A TNM-based stage grouping of I–IV is a prognostic indicator in patients with lung cancer. However, TNM staging is not able to differentiate between stage III/IV patients with oligometastatic disease and patients with extensive metastatic NSCLC. In the routine clinical setting, the prognosis of stage IV NSCLC that presents as oligometastatic disease may not differ significantly from that of stage III NSCLC(3). In parallel with the TNM staging, metabolic parameters on 18F-fluorodeoxyglucose positron-emission tomography/computed tomography (18F-FDG PET/CT)—maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG)—have been shown to be predictors of the risk of disease recurrence and death in patients with NSCLC and may be used in order to stratify such patients; those who are at a higher risk of recurrence or death may be candidates for treatments that are more aggressive(4). However, the SUVmax provides information only on a single pixel within the tumor, rather than measuring the volume or heterogeneity of the metabolically active disease(5). In patients with NSCLC in various stages, the analysis of SUVmax in conjunction with the volumetric parameters (MTV and TLG) has been shown to have greater prognostic value than does that of SUVmax alone(4). The addition of the volumetric parameters improves the determination of the biologically relevant lesion volume, especially in NSCLC(6). Although the volumetric parameters obtained from 18F-FDG PET/CT studies are especially significant prognostic factors for outcomes in patients with TNM stage I or II NSCLC(7–9), the application of such parameters in advanced disease has not been extensively studied(10). As previously mentioned, TNM staging is not able to differentiate between patients with advanced (stage III or IV) oligometastatic NSCLC and those with extensive metastatic NSCLC. Therefore, our study aimed to determine whether the 18F-FDG PET/CT-determined whole-body tumor burden is a better prognostic indicator than is the TNM stage in patients with advanced NSCLC, specifically whether it can discriminate between the prognosis of patients with oligometastatic stage IV disease and that of those with extensive locoregional stage III disease. MATERIALS AND METHODS Patients This was a prospective study in which we evaluated baseline 18F-FDG-PET/CT scans performed for staging between March 2016 and September 2017. The local institutional review board approved the study (Reference no. 53952516.1.0000.5404). All participating patients and professionals gave written informed consent. All patients with histologically confirmed stage III or IV NSCLC were included in the analysis, as were those in whom the histopathology was not able to specify the type of NSCLC. All of the patients underwent standard treatment with platinum-based chemotherapy, with or without radiotherapy (included only for patients with stage III disease), with subsequent clinical follow-up. Patients under 18 years of age were excluded, as were those who had had another neoplasm (except nonmelanoma skin cancer) in the last five years and those with uncontrolled diabetes mellitus, as well as women who were pregnant or lactating. 18F-FDG-PET/CT acquisition All patients fasted for 6 h before undergoing 18F-FDG-PET/CT, which involved the injection of 4.44 MBq/kg of 18F-FDG. In all cases, the fasting serum glucose level was below 180 mg/dL. The studies were performed in a 64-slice PET/CT scanner (Biograph TruePoint 64; Siemens Medical Solutions, Knoxville, TN, USA) 60 min after radiotracer injection. The CT parameters included 5 mm axial reconstruction, with a tube voltage of 120 kV or automatic tube voltage selection (CARE Dose 4D; Siemens Healthcare, Forchheim, Germany). The PET images were acquired from the head to upper thighs in three-dimensional mode with 90 s/bed position, without contrast administration. Image analysis and parameters used for quantification Two experienced observers, working independently, performed the quantitative visual analyses of all images. The final quantification was defined by consensus. On 18F-FDG PET/CT, the semiquantitative parameters evaluated were whole-body SUVmax (wbSUVmax, obtained from all metastatic sites including the primary lesion) and SUVmax of the primary tumor (tuSUVmax), whereas the quantitative parameters were whole-body MTV (wbMTV, including all metastatic sites and the primary lesion), primary tumor MTV (tuMTV), whole-body TLG (wbTLG, including all metastatic sites and the primary tumor), and primary tumor TLG (tuTLG), as depicted in Figure 1. We also calculated the wbSUVmax/tuSUVmax, wbMTV/tuMTV, and wbTLG/tuTLG ratios. 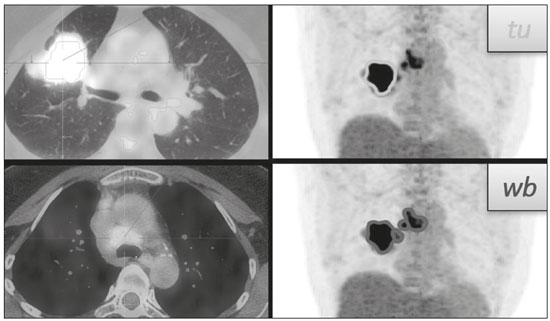 Figure 1. 18F-FDG PET/CT in a patient with advanced NSCLC, showing the VOIs for the tuTLG (upper) and wbTLG (lower). All semi-automated assessments were performed with a semi-automated multifocal segmentation tool (Syngo.via MM Oncology; Siemens Medical Solutions, Knoxville, TN, USA). We established a three-dimensional isocontour threshold of 50% of the single-pixel SUVmax to delineate the tumor contours with a lower-limit cutoff SUVmax of 2.5 to allow the software to calculate tumor burden of the primary and metastatic lesions(11–13). After the threshold selections were established, the software automatically generated volumes of interest (VOIs) for any structure with an SUV greater than the pre-established SUVmax threshold selection. It was necessary, at this point, to verify that every VOI delineated corresponded to a tumor site; if not, the VOI in question was manually excluded. Subsequently, the tumor burden was determined according to the selected tumor-related areas and the software automatically provided the 18F-FDG PET/CT parameters for the whole-body tumor burden. Statistical analyses Statistical analyses were performed with the SAS System, version 9.4 for Windows (SAS Institute Inc., Cary, NC, USA). Cox regression analysis examined the effects that the whole-body tumor burden parameters and other clinical and pathological variables—age, sex, Eastern Clinical Oncology Group (ECOG) performance status, histology, and TNM stage—had on OS and progression-free survival (PFS). The correlation between wbMTV and wbTLG was analyzed with Spearman’s correlation coefficient. Initially, the individual effects of variables on the outcomes were examined by univariate analyses. Subsequently, a forward and backward stepwise procedure was performed, and variables with p-values < 0.05 were selected for the multivariate analysis. Values of p ≤ 0.05 were considered statistically significant. RESULTS We evaluated 52 baseline 18F-FDG PET/CT scans performed for staging in 52 patients (mean age, 64.2 years; 24 women and 28 men). The median and mean follow-up times were 11.0 and 11.7 months, respectively, and the estimated median PFS and OS were 9.6 and 11.6 months, respectively. None of the patients were lost to follow-up. During follow-up, there was disease progression in 31 (59.7%) of the patients and 26 (50.0%) died. Table 1 shows the demographic and clinical characteristics of all patients; Table 2 describes the PFS and OS data; and Table 3 shows the distribution of the SUV, MTV, and TLG parameters. 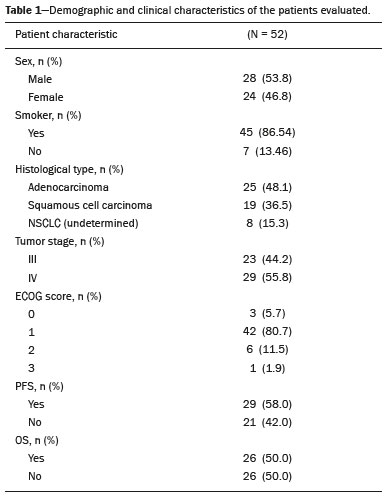 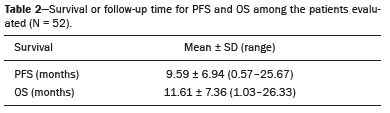 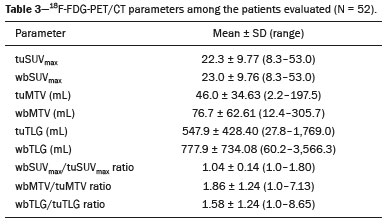 To evaluate the effects that the tuSUVmax, wbSUVmax, wbSUVmax/tuSUVmax ratio, tuMTV, wbMTV, wbMTV/tuMTV ratio, tuTLG, wbTLG, wbTLG/tuTLG ratio, performance status, and clinical stage have on OS, we applied a univariate Cox proportional-hazards model, including all of the same variables as the full model, and subsequently used a forward stepwise selection to construct the final model. The multivariate analysis included only the variables that achieved significance in the univariate analysis. In the univariate analysis, OS was found to correlate significantly with the wbMTV (hazard ratio [HR] = 1.008; 95% CI: 1.002–1.014; p = 0.0076), wbTLG (HR = 1.001; 95% CI: 1.000–1.001; p = 0.0361), and wbTLG/tuTLG ratio (HR = 1.705; 95% CI: 1.232–2.362; p = 0.0013). Because all MTV and TLG parameters are sub-products of each other and therefore strongly associated (p < 0.0001), only TLG-based parameters were included in the multivariate analyses. In the multivariate analysis, only the wbTLG/tuTLG ratio was independently associated with OS (HR = 1.660; 95% CI: 1.193–2.310; p = 0.0027). None of the other tumor burden parameters (wbSUVmax, tuSUVmax, tuMTV, tuTLG, wbSUVmax/tuSUVmax ratio, or wbMTV/tuMTV ratio), sex, age, performance status, histological subtype, TNM stage, or treatment type was found to be associated with PFS or OS in patients with advanced NSCLC (Table 4). 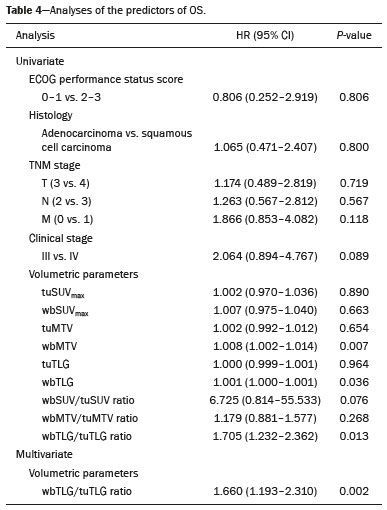 DISCUSSION In this prospective study, we have demonstrated that, in patients with advanced (stage III or IV) NSCLC, the whole-body metabolic tumor burden determined on a baseline 18F-FDG PET/CT performed for staging seems to be a strong, independent imaging biomarker to predict OS, better than the clinical evaluation of the primary tumor itself. The wbTLG/tuTLG ratio was found to be the best predictor of OS in our patient sample. In advanced NSCLC, the metabolism of the primary lesion, the TNM stage, and clinical parameters, although useful in the overall risk stratification, does not reflect the differences in survival when discriminating between patients with extensive metastatic disease and those with oligometastatic disease. The wbTLG/tuTLG ratio has the advantage of reflecting the relationship between and comparing the overall burden of disease and that of the primary tumor. Therefore, it seems reasonable to believe that this ratio reflects the aggressiveness of the disease because it reflects the capability of the tumor to spread in comparison with the burden of the primary lesion. In advanced NSCLC, a part of the tumor load, or even the primary tumor burden, is due to metastases, and the primary lesion alone (tuTLG) therefore does not adequately demonstrate the actual tumor burden, as demonstrated in Figure 2. 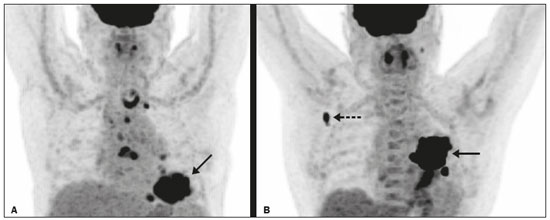 Figure 2. Examples of the importance of the tumor burden, calculated from 18F-FDG PET/CT imaging, as a prognostic indicator. A: A patient with stage IIIB NSCLC. The primary tumor (arrow) shows intense uptake (SUV = 13.2), and the multiple mediastinal lymph node metastases have intense hypermetabolism. B: A patient with stage IV NSCLC. The primary tumor (arrow) shows intense uptake (SUV = 47.2), and the patient was classified as having stage IV disease due to a contralateral axillary lymph node metastasis (dotted arrow). According to the TNM staging, the estimated 5-year OS rate was 19% for the patient depicted in A and 6% for the patient depicted in B. The wbTLG/tuTLG ratio was 1.27 for the patient in A and 1.01 for the patient in B. The patient in A had a higher tumor burden and died 11.57 months after diagnosis, whereas the patient in B, despite having been classified as having stage IV disease, was alive 25.7 months after diagnosis. Therefore, the wbTLG/tuTLG ratio, which is an independent prognostic indicator, may be a more accurate tool for assessing OS, which is especially useful in oligometastatic NSCLC. In our study, the univariate analyses showed that wbTLG and wbMTV were highly significant predictors of OS (p < 0.05 for both). Our findings corroborate those of other studies in which patients with a high MTV or TLG were found to be at a higher risk of adverse events and death(14). Therefore, the volumetric parameters are more reliable parameters(15) and should be used, as complements to the SUV, as incremental predictors of survival and events in patients with advanced NSCLC(8,14). However, in the present study, they were not strong independent predictors, most likely because of the small number of patients evaluated, the narrow confidence intervals, and the low HRs. As in other studies of patients with NSCLC(9,16), the majority of our patients were male and adenocarcinoma was the predominant histological type(15,17). However, unlike what has been reported in most such studies, in which the performance status of the patients is typically good (ECOG score = 0), nearly 92% of our patients had an ECOG score of 1 or 2, because they all had advanced NSCLC, which could account for the fact that there was no significant difference between those with stage III disease and those with stage IV disease. As expected on the basis of the literature(9), age, performance status, histological subtype, TNM stage, and treatment type were not predictive of PFS or OS in our sample of patients with advanced NSCLC. Likewise, another study of patients with NSCLC showed that TLG was associated with both PFS and OS more strongly than were other variables, such as smoking status, performance status, and histological type, which have previously been reported to correlate significantly with patient survival(18). In most of our patients, the SUVmax was highest in the primary lung lesion, being highest in metastatic lesions in only a few cases, the tuSUVmax and wbSUVmax therefore being coincident (given that the wbTLG includes the primary lesion). Analysis of the SUV in isolation has limitations. Isolated SUVs may vary according to blood glucose level, fasting time, and uptake time, as well as the methods employed for attenuation correction and reconstruction. The clinical importance of SUVmax as a prognostic factor is still unclear(8). In advanced NSCLC, FDG uptake in the primary tumor alone does not correlate significantly with survival(5,9,14). However, a meta-analysis including a collective total of 5,807 surgical patients with NSCLC demonstrated that high SUVmax, MTV, and TLG predicted a higher risk of recurrence or death(19). In contrast, another meta-analysis showed that the pre-radiotherapy and post-radiotherapy primary tumor SUVmax could predict the outcome of NSCLC patients treated with radiotherapy, because patients with a higher pre-radiotherapy SUVmax seemed to have a shorter OS and less local control(20). Although some of our patients underwent radiotherapy, we did not find a correlation between the pre-radiotherapy SUVmax and survival. The present study involved the use of semi-automated observer-independent software and, unlike most studies on this topic, had a prospective design. However, it has some limitations. First, the lesions were defined by using a threshold method. The choice of the threshold may influence the measurement of tumor volume, the mean SUV, and the wbTLG. We used 50% of the SUVmax, which is a commonly adopted threshold(9,21,22), to determine the tumor volume and then evaluated the results with the fused CT images to decide if further adjustment of the threshold was needed. Therefore, although the method is semi-automated, regions of physiological uptake were manually excluded. In addition, because this was a prospective study, it had a relatively small number of patients and a relatively short follow-up period. Furthermore, although all of our patients underwent chemotherapy, only those with stage III disease also underwent radiotherapy. Therefore, PFS and OS may also have been influenced by the treatment strategy employed. Moreover, the wbTLG/tuTLG ratio is new in the literature and has some intrinsic limitations, mainly the inability to differentiate between a high primary tumor burden with a high metastatic tumor burden and a low primary tumor burden with a low metastatic tumor burden. Further studies are needed in order to determine standard cutoff values and delineation methods for predicting prognosis using this parameter. The wbTLG/tuTLG ratio should be tested in other advanced malignant FDG-avid neoplasms to prove its utility. CONCLUSION In patients with advanced (stage III or IV) NSCLC, the wbTLG/tuTLG ratio measured on baseline 18F-FDG PET/CT performed for staging seems to be a strong, independent imaging biomarker, better than evaluation of the primary tumor in isolation, to predict OS. Acknowledgments We are grateful for the invaluable contributions of the following investigators from the São Paulo State University at Campinas, in Campinas, Brazil: Drs. Paulo Fanti and Cleide Moreira Silva (Department of Statistics); Drs. Eduardo Baldon Pereira and Antonio Carlos Zuliani (Division of Radiotherapy of the Department of Radiology); and Dr. Lair Zambon (Division of Pulmonology of the Department of Internal Medicine). REFERENCES 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. 2. American Cancer Society. Key statistics for lung cancer. [cited 2020 Aug 12]. Available from: https://www.cancer.org/cancer/lung-cancer/ about/key-statistics.html. 3. Couñago F, Luna J, Guerrero LL, et al. Management of oligometastatic non-small cell lung cancer patients: current controversies and future directions. World J Clin Oncol. 2019;10:318–39. 4. UyBico SJ, Wu CC, Suh RD, et al. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics. 2010;30:1163–81. 5. Hoang JK, Hoagland LF, Coleman RE, et al. Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol. 2008;26:1459–64. 6. Davison J, Mercier G, Russo G, et al. PET-based primary tumor volumetric parameters and survival of patients with non-small cell lung carcinoma. AJR Am J Roentgenol. 2013;200:635–40. 7. Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38. 8. Satoh Y, Onishi H, Nambu A, et al. Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology. 2014;270:275–81. 9. Chen HHW, Chiu NT, Su WC, et al. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology. 2012;264:559–66. 10. Burger IA, Casanova R, Steiger S, et al. 18F-FDG PET/CT of non-small cell lung carcinoma under neoadjuvant chemotherapy: background-based adaptive-volume metrics outperform TLG and MTV in predicting histopathologic response. J Nucl Med. 2016;57:849–54. 11. Boellaard R, Delgado-Bolton R, Oyen WJG, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. 12. Houdu B, Lasnon C, Licaj I, et al. Why harmonization is needed when using FDG PET/CT as a prognosticator: demonstration with EARL-compliant SUV as an independent prognostic factor in lung cancer. Eur J Nucl Med Mol Imaging. 2019;46:421–8. 13. Salaün PY, Abgral R, Malard O, et al. Good clinical practice recommendations for the use of PET/CT in oncology. Eur J Nucl Med Mol Imaging. 2020;47:28–50. 14. Im HJ, Pak K, Cheon GJ, et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:241–51. 15. Zaizen Y, Azuma K, Kurata S, et al. Prognostic significance of total lesion glycolysis in patients with advanced non-small cell lung cancer receiving chemotherapy. Eur J Radiol. 2012;81:4179–84. 16. Chung HW, Lee KY, Kim HJ, et al. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol. 2014; 140:89–98. 17. Ma W, Wang M, Li X, et al. Quantitative 18F-FDG PET analysis in survival rate prediction of patients with non-small cell lung cancer. Oncol Lett. 2018;16:4129–36. 18. Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013;14:1–12. 19. Liu J, Dong M, Sun X, et al. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One. 2016;11:e0146195. 20. Na F, Wang J, Li C, et al. Primary tumor standardized uptake value measured on F18-fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non-small-cell lung cancer receiving radiotherapy: meta-analysis. J Thorac Oncol. 2014;9:834–42. 21. Konert T, Vogel WV, Paez D, et al. Introducing FDG PET/CT-guided chemoradiotherapy for stage III NSCLC in low- and middle-income countries: preliminary results from the IAEA PERTAIN trial. Eur J Nucl Med Mol Imaging. 2019;46:2235–43. 22. Mac Manus MP, Hicks RJ. The role of positron emission tomography/computed tomography in radiation therapy planning for patients with lung cancer. Semin Nucl Med. 2012;42:308–19. Faculdade de Ciências Médicas da Universidade Estadual de Campinas (FCM-Unicamp), Campinas, SP, Brazil a. https://orcid.org/0000-0003-0620-6870 b. https://orcid.org/0000-0002-9192-6946 c. https://orcid.org/0000-0003-4839-6465 d. https://orcid.org/0000-0001-5570-197X e. https://orcid.org/0000-0001-6498-1758 f. https://orcid.org/0000-0003-4079-9577 g. https://orcid.org/0000-0003-4973-7591 h. https://orcid.org/0000-0002-8632-5943 Correspondence: Dr. Elba Cristina Sá de Camargo Etchebehere Setor de Medicina Nuclear, Departamento de Radiologia, Universidade Estadual de Campinas Cidade Universitária Zeferino Vaz, Barão Geraldo Campinas, SP, Brazil, 13083-970 Email: elba@hc.unicamp.br Received 8 May 2020 Accepted after revision 1 September 2020 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554