Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 53 nº 2 - Mar. / Apr. of 2020
Vol. 53 nº 2 - Mar. / Apr. of 2020
|
ADVANCES IN RADIOLOGY
|
|
Computed tomography-guided percutaneous neurolysis of celiac plexus: technical description |
|
|
Autho(rs): Renata Motta Gruberta; Tiago Kojun Tibanab; Larissa Araújo Missirianc; Thaline Mairace Hernandez das Nevesd; Thiago Franchi Nunese |
|
|
INTRODUCTION
Abdominal pain is a significant debilitating problem that is common in cancer patients, dramatically affecting quality of life and survival(1,2). The pain, which originates from the viscera of the upper abdomen, is transmitted by visceral afferent fibers that relay the impulses through the splanchnic nerves and the celiac plexus. As illustrated in Figure 1A, the celiac plexus is a network of nerve fibers located in the retroperitoneum along the anterolateral wall of the abdominal aorta(2,3). 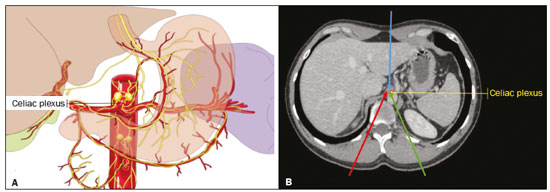 Figure 1. A: Illustration showing the location of the celiac plexus. B: Axial CT scan showing some approaches: anterior hepatic (blue arrow), posterior intervertebral (red arrow), posterior aortic (green arrow). The management of cancer-related abdominal pain is complex and challenging, often requiring the chronic use of high doses of opioids, which in turn are generally associated with several adverse effects(1,2). Although therapies employing imaging-guided percutaneous methods reduce the need for potent analgesics and the associated toxicity, such procedures are not widely disseminated and are underused(1,4–8). Celiac plexus neurolysis (CPN) is a technique that provides permanent interruption of plexus pain transmission through chemical ablation, potentially improving pain control while dramatically reducing opioid consumption(9,10). It involves the infusion of a neurolytic agent, typically sterile absolute alcohol, through a fine needle inserted into the retroperitoneum, adjacent to nerve fibers and the ganglia of the celiac plexus. The neurolytic agent disrupts the neural network, interrupting the pain pathways(2,11). Imaging guidance for CPN is most often performed by computed tomography (CT), which has replaced fluoroscopy and ultrasound for that purpose(2,12). PROCEDURE The first step in CT-guided NPC is pre-procedure planning. Preoperative images should be reviewed in detail to determine the positioning of the patient, as well as to select the puncture site, needle path, and neurolytic injection site (Figure 1B). The pre-procedure planning ensures that the agent is properly distributed, increases the analgesic effect, and reduces morbidity. Proper patient positioning is essential for a successful procedure, because it not only allows a safe percutaneous path to be mapped but also ensures patient comfort. Various approaches to CPN can be taken, including anterior and posterior approaches, the bilateral posterior paravertebral approach being the one most frequently employed (Figure 2A). Local anesthesia at the puncture site is performed under sedation with a benzodiazepine or an opioid, and oxygen is delivered via a nasal cannula. 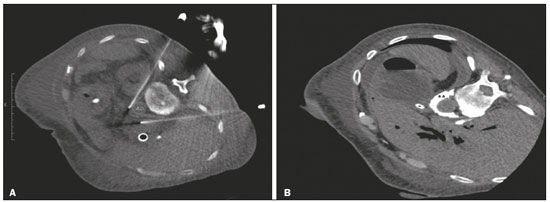 Figure 2. A: Axial CT scan, with the patient in the supine oblique position, showing bilateral paravertebral posterior punctures, adjacent to the aorta (Ao), at the level of the celiac trunk, made with a 22G Chiba needle. B: Correct distribution of absolute alcohol solution and iodinated contrast (asterisks). After CT-guided puncture and verification of the correct positioning of the needle(s), with or without injection of 1–2 mL of iodinated contrast agent, a total volume of 40–60 mL of the neurolytic agent (absolut alcohol solution) is infused (Figure 2B). At our facility, we also administer 2–3 mL of 1% lidocaine, without a vasoconstrictor, before and after injection of the alcohol. As a tool for palliative pain management, CPN is safe and effective, with a relatively low complication rate. It should be offered to patients as a key component of a multidisciplinary approach to the control of intractable chronic abdominal pain. Proper use and knowledge of imaging examinations and of the technique are invaluable to ensuring satisfactory results. REFERENCES 1. Sindt JE, Brogan SE. Interventional treatments of cancer pain. Anesthesiol Clin. 2016;34:317–39. 2. Kambadakone A, Thabet A, Gervais DA, et al. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics. 2011;31:1599–621. 3. Cornman-Homonoff J, Holzwanger DJ, Lee KS, et al. Celiac plexus block and neurolysis in the management of chronic upper abdominal pain. Semin Intervent Radiol. 2017;34:376–86. 4. Nunes TF, Tibana TK, Santos RFT, et al. Percutaneous insertion of bilateral double J stent. Radiol Bras. 2019;52:104–5. 5. Tibana TK, Grubert RM, Santos RFT, et al. Percutaneous nephrostomy versus antegrade double-J stent placement in the treatment of malignant obstructive uropathy: a cost-effectiveness analysis from the perspective of the Brazilian public health care system. Radiol Bras. 2019;52:305–11. 6. Nunes TF, Tibana TK, Pereira MES, et al. Treatment of extrahepatic biliary fistulas using n-butyl cyanoacrylate. Radiol Bras. 2019; 52:174–5. 7. Meira MS, Barbosa PNVP, Bitencourt AGV, et al. Retrospective analysis of computed tomography-guided percutaneous nephrostomies in cancer patients. Radiol Bras. 2019;52:148–54. 8. Falsarella PM, Rocha RD, Rahal Junior A, et al. Minimally invasive treatment of complex collections: safety and efficacy of recombinant tissue plasminogen activator as an adjuvant to percutaneous drainage. Radiol Bras. 2018;51:231–5. 9. Wyse JM, Chen YI, Sahai AV. Celiac plexus neurolysis in the management of unresectable pancreatic cancer: when and how? World J Gastroenterol. 2014;20:2186–92. 10. Sachdev AH, Gress FG. Celiac plexus block and neurolysis: a review. Gastrointest Endosc Clin N Am. 2018;28:579–86. 11. Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995; 80:290–5. 12. Wang PJ, Shang MY, Qian Z, et al. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging. 2006;31:710–8. Hospital Universitário Maria Aparecida Pedrossian da Universidade Federal de Mato Grosso do Sul (HUMAP-UFMS), Campo Grande, MS, Brazil a. https://orcid.org/0000-0001-6713-2575 b. https://orcid.org/0000-0001-5930-1383 c. https://orcid.org/0000-0001-5688-1028 d. https://orcid.org/0000-0002-6172-5465 e. https://orcid.org/0000-0003-0006-3725 Correspondence: Dr. Tiago Kojun Tibana Avenida Senador Filinto Müller, 355, Vila Ipiranga Campo Grande, MS, Brazil, 79080-190 Email: tiagotibana@hotmail.com Received 16 January 2019. Accepted after revision 22 February 2019. |
|
GN1© Copyright 2024 - All rights reserved to Colégio Brasileiro de Radiologia e Diagnóstico por Imagem
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554