Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 52 nº 4 - July / Aug. of 2019
Vol. 52 nº 4 - July / Aug. of 2019
|
LETTERS TO THE EDITOR
|
|
Subacute cortical infarct: the value of contrast-enhanced FLAIR images in inconclusive DWI |
|
|
Autho(rs): Pantelis Kraniotis1; Aikaterini Solomou2 |
|
|
Dear Editor,
A 44-year-old patient presented with axial sensorimotor deficit, dating back approximately 10 days. The history was significant for diabetes, alcoholism, and cognitive impairment, making it difficult to assess the recent history and symptoms. The patient was submitted to brain magnetic resonance imaging (MRI) with T2-weighted imaging (T2WI), fluid-attenuated inversion recovery (FLAIR) sequences, susceptibility weighted imaging, and diffusion-weighted imaging (DWI), as well as T1-weighted imaging (T1WI), before and after intravenous gadolinium administration, in the axial, sagittal, and coronal planes. In the right postcentral gyrus, MRI revealed a cortical lesion, which showed a hyperintense signal on FLAIR image (Figure 1A). The lesion could be due to a new or older infarct. However, there was no restricted diffusion suggestive of a recent infarct (Figures 1B and 1C). Contrast-enhanced FLAIR imaging revealed marked cortical enhancement in the right postcentral gyrus, consistent with a subacute cortical infarct (Figure 1D). 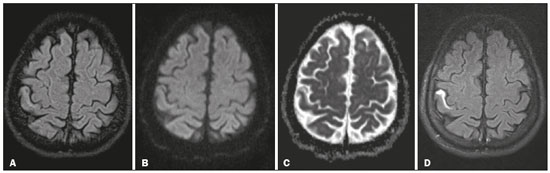 Figure 1. A: Axial FLAIR image. Note the subtly hyperintense signal in the anterior cortex of the postcentral gyrus. B,C: Corresponding axial DWI (B) and ADC map (C). There is a barely discernible hyperintense signal on DWI, although there is no evidence of low signal intensity on the ADC map (i.e., there is no restricted diffusion in the region). D: Gadolinium-enhanced FLAIR image showing marked contrast uptake in the affected area of the postcentral gyrus. Diabetes mellitus is a well-recognized risk factor for ischemic stroke, which is a leading cause of death and disability. MRI is quite sensitive in detecting ischemic changes. T2WI is more sensitive than is T1WI, and T1WI after gadolinium administration can provide valuable information for the accurate diagnosis(1,2). Intravascular enhancement, although not specific, is considered a sign of ischemia on conventional MRI. Contrast enhancement in the central nervous system is the result of a combination of disruption of the blood-brain barrier, high vascularity, and contrast leakage into the lymphatic system(3–6). After one week, infarcts show parenchymal enhancement, due to breakdown of the blood-brain barrier(7). New imaging techniques, such as DWI and perfusionweighted imaging, have increased the accuracy of the diagnosis of acute cerebral ischemia, although there are some cases in which it cannot be distinguished from other entities(8,9). In addition, because of pseudonormalization, subacute infarcts may not show restricted diffusion on DWI. FLAIR is highly sensitive for the detection of ischemic lesions. Although it is considered to be heavily T2-weighted, rendering cerebrospinal fluid as dark, it also shows mild contrast enhancement on T1WI, which is responsible for the increased conspicuity of gadolinium enhancement. Pathologic conditions that present contrast enhancement on T1WI usually show marked enhancement on contrast-enhanced FLAIR(10). This is exactly what occurred in the case presented here, in which DWI pseudonormalization did not help reveal the subacute cortical infarct. When a subacute cortical infarct is suspected, delayed contrast-enhanced FLAIR imaging is the best choice for demonstrating the lesion and for differentiating it from an older lesion with gliosis. REFERENCES 1. Yuh WT, Crain MR, Loes DJ, et al. MR imaging of cerebral ischemia: findings in the first 24 hours. AJNR Am J Neuroradiol. 1991;12:621–9. 2. Weinmann HJ, Brasch RC, Press WR, et al. Characteristics of gadolinium- DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol. 1984;142:619–24. 3. Bozzao A, Floris R, Fasoli F, et al. Cerebrospinal fluid changes after intravenous injection of gadolinium chelate: assessment by FLAIR MR imaging. Eur Radiol. 2003;13:592–7. 4. Fukuoka H, Hirai T, Okuda T, et al. Comparison of the added value of contrast-enhanced 3D fluid-attenuated inversion recovery and magnetization- prepared rapid acquisition of gradient echo sequences in relation to conventional postcontrast T1-weighted images for the evaluation of leptomeningeal diseases at 3T. AJNR Am J Neuroradiol. 2010;31:868–73. 5. Sage MR, Wilson AJ, Scroop R. Contrast media and the brain. The basis of CT and MR imaging enhancement. Neuroimaging Clin N Am. 1998;8:695–707. 6. Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27:525–51. 7. Karonen JO, Partanen PL, Vanninen RL, et al. Evolution of MR contrast enhancement patterns during the first week after acute ischemic stroke. AJNR Am J Neuroradiol. 2001;22:103–11. 8. Beaulieu C, de Crespigny A, Tong DC, et al. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol. 1999;46:568–78. 9. Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology. 1999;210:519–27. 10. Lee EK, Lee EJ, Kim S, et al. Importance of contrast-enhanced fluidattenuated inversion recovery magnetic resonance imaging in various intracranial pathologic conditions. Korean J Radiol. 2016;17:127–41. 1. University General Hospital of Patras, Patras, Greece; https://orcid.com/0000-0001-9149-1586 2. University General Hospital of Patras, Patras, Greece; https://orcid.com/0000-0002-8501-1192 Correspondence: Pantelis Kraniotis, MD University General Hospital of Patras Hippokratous av., Patras 265 00, Greece Email: pantelis.kraniotis@gmail.com Received 16 October 2017 Accepted after revision 27 November 2017 |
|
GN1© Copyright 2025 - All rights reserved to Colégio Brasileiro de Radiologia e Diagnóstico por Imagem
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554