Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 52 nº 1 - Jan. /Feb. of 2019
Vol. 52 nº 1 - Jan. /Feb. of 2019
|
ORIGINAL ARTICLES
|
|
Is interim 18F-fluoride PET/CT a predictor of outcomes after radium-223 therapy? |
|
|
Autho(rs): Elba Etchebehere1,a; Ana Emília Brito2,b; Kalevi Kairemo3,c; Eric Rohren4,d; John Araujo5; Homer Macapinlac6 |
|
|
Keywords: Sodium fluoride; Positron-emission tomography/methods; Tomography, X-ray computed/methods; Prostatic neoplasms; Radium-223; Bone neoplasms/diagnostic imaging; Tumor burden. |
|
|
Abstract: INTRODUCTION
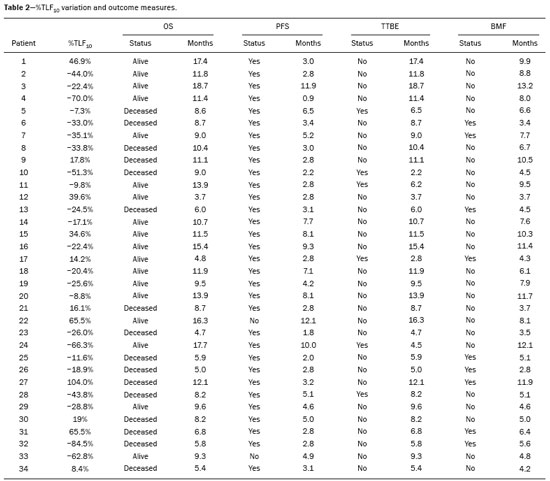
Baseline whole-body 18F-fluoride PET/CT is ideal for staging and restaging prostate cancer and has been shown to be an independent prognostic imaging biomarker of patients undergoing radium-223 dichloride (223RaCl2) therapy(1). However, although treatment with 223RaCl2 improves survival in prostate cancer patients(2–4), not all patients respond to this therapy. It would be beneficial to identify nonresponders early in the course of treatment, thereby reducing morbidity and unnecessary costs. After successful treatment of osteoblastic bone metastases, an osteoblastic reaction (flare) can occur, which increases bone uptake even in responsive cases. That can be confused with the osteoblastic reaction and inflammation that occur in response to tumor-associated growth factors during progression. This phenomenon has been well described in conventional bone scintigraphy, and that method is therefore not recommended for use as the sole means of determining the response to treatment(5,6). Although interim studies performed with 18F-FDG PET/CT can change the management of patients with a variety of cancer types(7–10), the exact role of 18F-fluoride PET/CT in evaluating the early response to therapy (interim study) is not well established. The importance of 18F-fluoride PET/CT has extended beyond the diagnosis of metastases to the evaluation of optimal strategies for use in patients submitted to treatment with new therapeutic agents. Chemotherapy, hormone therapy, immunotherapy, and radionuclide therapies such as those involving 223RaCl2(11) are costly approaches. Therefore, the ability to predict response, thereby avoiding overtreatment and reducing costs, will improve patient management. The purpose of this study was to determine whether an interim 18F-fluoride PET/CT study is able to evaluate treatment responses in prostate cancer patients submitted to 223RaCl2 therapy. MATERIALS AND METHODS The local institutional review board approved this retrospective analysis (reference no. PA14-0848). We retrospectively reviewed histologically confirmed cases of hormone-refractory prostate cancer with bone metastasis in patients receiving 223RaCl2 therapy and undergoing two 18F-fluoride PET/CT studies—a baseline study and an interim study (immediately prior to the fourth cycle of 223RaCl2)—at our facility. All patients completed at least four cycles of 223RaCl2 (Xofigo; Bayer Pharma AG, Berlin, Germany), receiving intravenous infusions of 50 kBq/kg (1.4 µCi/kg) of 223RaCl2 at monthly intervals. 18F-fluoride PET/CT acquisition 18F-fluoride PET/CT images were acquired immediately prior to initiation of the 223RaCl2 therapy (baseline study) and immediately before the fourth cycle (interim study). True whole-body PET images were obtained 50–60 min after intravenous injection of 158–370 MBq of 18F-sodium fluoride in dedicated PET/CT scanners (Discovery STe, RX, or VCT; 16 or 64 channel; GE Healthcare, Milwaukee, WI, USA, or Siemens mCT Flow; 64 channel; Siemens Healthcare, Knoxville, TN, USA), and whole-body noncontrast CT scans were used for attenuation correction. 18F-fluoride PET/CT interpretation and quantification Two board-certified nuclear medicine physicians evaluated baseline and interim 18F-fluoride PET/CT images. Visual and quantitative analyses were performed. Visual analysis In the visual analysis, we compared the baseline and interim studies, classifying the responses as follows: • Complete response – Osteoblastic bone metastases identified in the baseline study no longer being present in the interim study. • Partial response – Interim study showing decreased uptake in pre-existing bone metastases. • Stable disease – No difference between the interim and baseline scans in terms of the uptake in pre-existing bone metastases. • Progressive disease – Interim study showing an increase in the uptake or volume of a pre-existing bone metastases or new osteoblastic metastases. The patients were followed for confirmation of these response classifications. The follow-up reference standards used in order to determine if the response classification was correct (i.e., to identify true-positive, true-negative, false-positive, and false-negative responses) included clinical parameters—such as clinical worsening, disease progression, bone events, and death; biochemical parameters—such as the levels of alkaline phosphatase (ALP) and prostate-specific antigen (PSA); and imaging findings—such as those obtained with 18F-fluoride PET/CT, 18F-FDG PET/CT, bone scans, or CT scans. On the interim 18F-fluoride PET/CT study, images that demonstrated stable disease, a partial response, or a complete response were all considered to represent a true-positive response to therapy if the reference standards also indicated that the patient had responded to therapy (no clinical worsening, progression, or increase in the levels of the biochemical markers) or a false-positive response to therapy if those same standards demonstrated progressive disease (new areas of disease, clinical worsening, or death). In contrast, images that demonstrated progressive disease were considered to represent a true-negative response to therapy if the reference standards also indicated that the patient had not responded to therapy and it was confirmed during follow-up that there was no response to therapy or a false-negative response to therapy (flare response) if those same standards demonstrated a response (no clinical worsening, progression, or increase in the levels of the biochemical markers). Quantitative analysis Using quantitative analysis, we determined the whole-body skeletal tumor burden in the baseline and interim 18F-fluoride PET/CT images. The skeletal tumor burden was determined after establishing the maximum standardized uptake value (SUVmax) threshold ≥ 10 to exclude normal bone(12). To that end, we initially obtained the volume (in milliliters) of total fluoride activity, defined as the fluoride tumor volume above an SUVmax of 10 (FTV10), within the volume of interest (VOI). The FTV10 calculation is equivalent to the metabolic tumor volume calculation used in 18F-FDG PET/CT studies. The total fluoride lesion uptake above an SUVmax of 10 (TLF10) was then calculated as the product of mean SUVmax × FTV10. The TLF10 is equivalent to the total lesion glycolysis used in 18F-FDG PET/CT studies. To evaluate the performance of interim 18F-fluoride PET/CT, the percent change in the skeletal tumor burden between the baseline and interim studies was calculated as follows: %TLF10 = interim TLF10 − baseline TLF10 / baseline TFL10 Statistical analyses Categorical variables were expressed as absolute and relative frequencies, whereas continuous variables were expressed as mean ± standard deviation when presenting normal distribution and as median (minimum-maximum) when presenting non-normal distribution. All outcome measures were correlated with the %TFL10 values obtained. The primary end point was overall survival (OS), which was calculated from the first 223RaCl2 cycle to the date of death or last follow-up. Secondary end points were progression-free survival (PFS), time to a bone event (TTBE), and bone marrow failure (BMF). PFS was calculated from the first 223RaCl2 cycle to the date of progression, death, or last follow-up. The TTBE was calculated as the time from the date of the first 223RaCl2 cycle to the next bone event. Lastly, BMF was defined as the development of hematologic toxicity (World Health Organization grade 3 or 4), together with no recovery after six weeks or death due to BMF after the last 223RaCl2 cycle. Kaplan-Meier survival curves were generated, and Cox proportional hazards regression was used in order to analyze predictors of survival. Backward stepwise selection was performed for multivariate Cox models. Logistic regression was used in order to model the odds of a bone event as a function of all of the PET variables. We used Spearman’s correlation coefficient to assess the level of agreement between the PET variables. For the statistical analyses, we used the Statistical Analysis System, version 9.3 for Windows (SAS Institute Inc., Cary, NC, USA). RESULTS We analyzed the cases of 34 patients, with a mean age of 72.4 ± 10.2 years (median, 72.5 years; range, 43.3–88.8 years) (Table 1), who had had prostate cancer for a mean of 6 ± 4 years (range, 2–20 years). The mean Gleason score was 7 ± 3. Prior to the initiation of 223RaCl2 therapy, 26.9% of the patients had received chemotherapy, 5% had received radiotherapy, 59% had received hormone therapy, and 9% had received blood transfusion. At the first 223RaCl2 cycle, the mean ALP was 193.9 IU/L and the mean PSA was 103.2 ng/mL. The median time of follow-up after the interim study was 28.1 months (range, 11–52 months). The 34 patients were submitted to a collective total of 179 223RaCl2 cycles: 55.9% of the patients received six cycles of 223RaCl2; 14.7% received five cycles; and 29.4% received four cycles. The principal causes of treatment interruption were progression (in 44.4%), hematologic toxicity (in 17.8%), a significant decline of the Eastern Cooperative Oncology Group performance status (in 13.3%), and a bone event (in 2.2%). 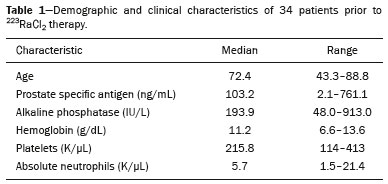 Visual analysis of interim 18F-fluoride PET/CT A complete response was not perceived in any of the interim 18F-fluoride PET/CT studies or on the basis of the follow-up reference standards. A partial response was identified in 16 (47%) of the patients in the interim 18F-fluoride PET/CT studies (Figure 1), and the reference standards demonstrated that a partial response had indeed been achieved in eight of those patients (true-positive cases), whereas the other eight patients had progressed (false-positive cases), as shown in Figure 2. Stable disease was noted in five (15%) of the patients in the interim 18F-fluoride PET/CT studies, although only three of those patients were categorized as true-positive cases (showing stable disease or a partial response), whereas the two remaining patients progressed. Progressive disease was identified in 13 (38%) of the patients in the interim 18F-fluoride PET/CT studies, 12 (35.3%) of whom were categorized as true-negative cases (Figure 3), the remaining patient (3.0%) being categorized as a false-negative case because the increased uptake noted on the interim 18F-fluoride PET/CT (when compared with that observed in the baseline study) was actually due to a flare phenomenon (Figure 4). Therefore, the responses were categorized as true positive in 11 cases (32.4%), false positive in 10 (29.4%), true negative in 12 (35.3%), and false negative in 1 (2.9%). 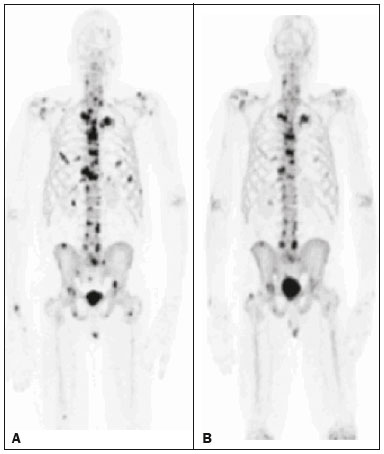 Figure 1. A patient with hormone-refractory prostate cancer, accompanied by bone metastasis, who showed a partial response to 223RaCl2, and the interim 18F-fluoride PET/CT study demonstrating a true-positive response. A: The baseline 18F-fluoride PET/CT study revealing widespread osteoblastic metastases. B: The interim 18F-fluoride PET/CT study, performed after the third 223RaCl2 cycle, showing a reduction in osteoblastic metastases, especially in the rib cage, pelvis, and right femur, consistent with a partial response to 223RaCl2. There was a 70% reduction in the %TLF10. During follow-up, the ALP levels dropped and no new bone lesions appeared. After the last 223RaCl2 cycle, the patient resumed enzalutamide to control lymph node metastases that had been present prior to the first 223RaCl2 cycle. 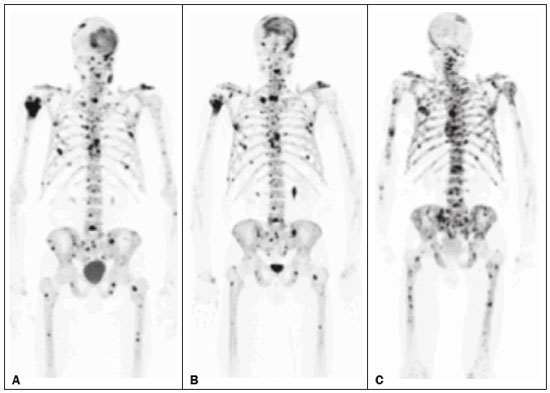 Figure 2. A patient with hormonerefractory prostate cancer, accompanied by bone metastasis, who showed progression during 223RaCl2 therapy but was categorized as a false-positive case on the basis of the imaging findings. A: The baseline 18F-fluoride PET/CT study showing osteoblastic metastases. B: The interim 18Ffluoride PET/CT study, performed after the third 223RaCl2 cycle, showing a slight reduction in uptake by the known osteoblastic metastases and no new lesions, consistent with a partial response. Although the %TLF10 decreased by 44%, the PSA and ALP levels continued to rise and there was rapid progression of the bone metastases. Therefore, the patient was started on cyclophosphamide and subsequently on dasatinib. C: A follow-up 18F-fluoride PET/CT study, conducted after the sixth 223RaCl2 cycle, showed wide-spread osteoblastic metastases. 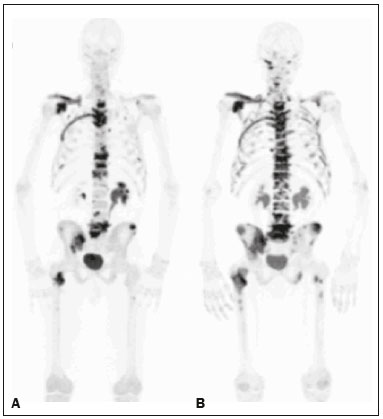 Figure 3. A patient with hormone-refractory prostate cancer, accompanied by bone metastasis, who showed progression during 223RaCl2 therapy. A: The baseline 18F-fluoride PET/CT study showing widespread osteoblastic metastases. B: The interim 18F-fluoride PET/CT study, performed after the third 223RaCl2 cycle, showing increased uptake in the known osteoblastic metastases and new lesions, especially in the pelvis, consistent with progression. The %TLF10 increased by 104%; PSA and ALP levels continued to rise; new bone metastases developed; a liver metastasis developed; and there was further enlargement of previously enlarged lymph nodes. The patient started a new chemotherapy regimen but died eight months after the last 223RaCl2 cycle. 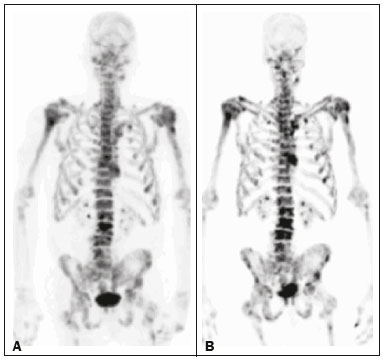 Figure 4. A patient with hormone-refractory prostate cancer, accompanied by bone metastasis, who responded to 223RaCl2 but was categorized as a falsenegative case on the basis of the imaging findings. A: The baseline 18F-fluoride PET/CT study showing osteoblastic metastases. B: The interim 18F-fluoride PET/CT study, performed after the third 223RaCl2 cycle, showing increased uptake in the known osteoblastic metastases but no new lesions. Although that pattern is consistent with progression (with a %TLF10 increase of 65%), the PSA and ALP dropped remarkably, after which the patient responded and was stable at 12 months after the last 223RaCl2 cycle. Therefore, the images were clearly due to a flare (false-negative) response. The interim 18F-fluoride PET/CT study was found to have a sensitivity of 91.6%, a specificity of 54.5%, a positive predictive value of 52.4%, a negative predictive value of 92.3%, and an accuracy of 67.6% (Figure 5). For distinguishing between responders and nonresponders, a reduction in the ALP level had a sensitivity of 38% and a specificity of 85% when the follow-up parameters were taken as the reference. 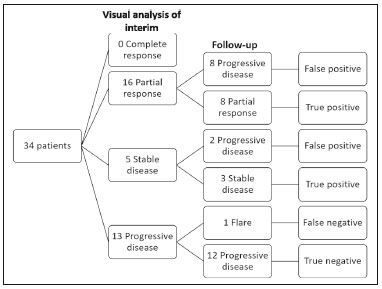 Figure 5. Visual analysis and evolution of the 34 patients. Quantitative analysis of interim 18F-fluoride PET/CT Figure 6 illustrates the quantitative method employed to obtain the TLF10 and FTV10 values. Spearman’s correlation coefficient showed that the %TLF10 and %FTV10 values correlated strongly with each other (rho = 0.95). Therefore, only the %TLF10 values were applied to subsequent analyses. The median TLF10 was 7374 (range, 391–46,550) in the baseline 18F-fluoride PET/CT study and 5632 (range, 486–30,200) in the interim study. 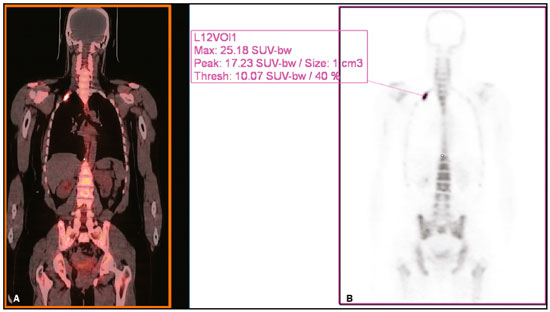 Figure 6. Example of determination of TLF10 and FTV10. A: A semi-automatic VOI (orange rectangle) is placed within the whole-body maximum intensity projection image. A threshold SUVmax of 10 is then established as the cut-off to separate normal bone from abnormal bone. Consequently, the software will automatically delineate only SUVmax regions above the set threshold of 10, defining the VOI with an isocontour threshold set at 41% of the SUVmax. After all regions have been defined, a careful inspection should be performed to exclude all non-tumor-related VOIs. The sum of all the VOIs outlined with the SUVmax of 10 provides the FTV10. To obtain the TLF10, the FTV10 is multiplied by the SUVmean10 (VOI10 × mean10), which is also measured in milliliters. B: In this particular example, the patient had only one lesion with an SUVmax higher than 10, which corresponded to a rib metastasis with an SUVmax of 25. The TLF10 was 65.8, and the FTV10 was 4.2. At the end of the follow-up period, 32 (94%) of the patients had progressed and 17 (53%) had died (Table 2). The average time to progression was 4.7 ± 2.9 months (median, 3.1 months; range, 0.9–12.1 months), and the most common type of progression was metastasis to the bone (in 39.1%), followed by nodal metastases (in 25.0%) and visceral metastases (in 21.9%). In our study sample, the %TLF10 on the interim 18F-fluoride PET/CT was not able to predict OS (p = 0.6320; HR = 0.753; 95% CI: 0.236–2.401) or PFS (p = 0.5908; HR = 1.248; 95% CI: 0.557–2.797). Six patients had a bone event, and %TLF10 was also unable to predict the TTBE (p = 0.5114; HR = 1.588; 95% CI: 0.399–6.312). Nine patients developed BMF after 223RaCl2, and %TLF10 was also not a significant univariate predictor of the odds of developing that condition (p = 0.6071; HR = 1.401; 95% CI: 0.387–5.070). We found that OS did not correlate with the SUVmax (p = 0.7989), any nodal disease (p = 0.1342), or visceral disease (p = 0.1496). DISCUSSION We have demonstrated that an interim 18F-fluoride PET/CT study is unable to predict outcomes after 223RaCl2 therapy. Novel therapies for osteoblastic metastases, including 223RaCl2 therapy, are costly, and it is therefore important to establish a diagnostic test to predict responses to these new, expensive treatments. In one study evaluating treatment responses after six cycles of 223RaCl2 in ten patients(13), conventional bone scintigraphy demonstrated that increased areas of uptake were due not only to treatment response but also to reparative bone changes after therapy (a flare response). Previous studies have shown that a baseline 18F-fluoride PET/CT study plays a prognostic role in patients with breast or prostate cancer treated with 223RaCl2(1,14). However, 18F-fluoride PET/CT is not traditionally used in evaluating the response to any therapy, because the process of bone healing involves an osteoblastic reaction than can increase 18F-fluoride uptake, as in conventional bone scintigraphy(15). Because of comparable pharmacokinetics between 223RaCl2(2) and 18F-fluoride(16), we hypothesized that 18F-fluoride would be able to evaluate osteoblastic metastases before, during, and after 223RaCl2 therapy. In our study sample, the interim study demonstrated that a decrease in uptake was generally due to a response (partial or stable disease) to therapy. However, we find it interesting that, in six (17.6%) of the patients, the decreased uptake was caused by extensive tumor infiltration of the bone marrow, ultimately leading to BMF. To our knowledge, there have been no previous studies describing the latter imaging pattern (caused by BMF) in interim studies. In contrast, although the interim study was able to demonstrate that increased uptake was due to progression, that pattern of uptake was in fact a flare phenomenon in one case. This increased uptake most likely occurred because of the bone healing process after successful 223RaCl2 treatment, which involves an osteoblastic reaction. In the subset of patients in which the flare phenomenon occurred, the CT portion of the study revealed reparative changes with increased extent of the sclerotic lesions. However, even on CT, it was not possible to determine which patients were progressing and which were responding. Although we hypothesized that bone levels of ALP could help evaluate patient outcomes, it demonstrated higher specificity and lower sensitivity than did the interim 18F-fluoride PET/CT study. Quantitative analyses of 18F-fluoride PET/CT images have been conducted to assess its role in predicting outcomes, by determining the peak SUVmax values of bone metastases. Apolo et al.(17) performed 18F-fluoride PET/CT after 6 and 12 months of standard therapy in prostate cancer patients, reporting that progression was associated with SUV increases of more than 57%, as well as that a greater increase in SUV was associated with worse survival. Yu et al.(18) evaluated responses to therapy with dasatinib using SUVmax in five target lesions on 18F-fluoride PET/CT and detected only a borderline correlation with PFS; the changes also correlated with the ALP level. Another study, involving only five patients, showed a reduction in SUVmax at 6 and 12 weeks after the use of 223RaCl2(19). In our population, the SUVmax did not correlate with OS. Although the above mentioned studies performed 18F-fluoride PET/CT for therapeutic evaluation, its precise role in determining the early response to therapy has yet to be extensively studied, especially in terms of assessing survival as an end point. The reported frequency of the flare phenomenon in prostate cancer patients undergoing conventional bone scintigraphy ranges from 6% to 25%(20,21). Although the flare phenomenon has also been described in patients undergoing 18F-fluoride PET/CT(15), there have been no reports of its frequency in patients treated with 223RaCl2 and undergoing 18F-fluoride PET/CT. Although we identified the flare phenomenon on 18F-fluoride PET/CT in only a small proportion of our patient sample (3%), that proportion is probably higher than in conventional bone scintigraphy, given the greater sensitivity of PET/CT. The most likely explanation for the fact that the frequency of the flare phenomenon was not higher is that our study sample was composed of patients with extensive disease, in whom the likelihood of progression is greater than is that of a response to therapy. In addition, the number of patients might have been insufficient to detect this phenomenon. In our patient sample, the interim 18F-fluoride PET/CT (%TLF10) after three cycles of 223RaCl2 was not able to predict OS, PFS, TTBE, or BMF. These findings are quite similar to those of a previous study, involving ten prostate cancer patients treated with 223RaCl2(22), although the interim 18F-fluoride PET/CT studies were performed at different time points: at baseline; after one (or two) cycles of 223RaCl2; and at the end of treatment. A correlation with outcome was only noted between baseline and end-of-treatment 18F-fluoride PET/CT results were found to correlate with outcomes, as previously reported(1). One major limitation of our study was the relatively small number of patients. We believe that the interim 18F-fluoride PET/CT could have potential for the prediction of BMF, given that 6 of the 9 patients who evolved to BMF showed a reduction in uptake. However, due to the small sample size, those results were not significant. Another limitation was the fact that it was not possible to obtain histological confirmation in the patients who showed progression. Although the 18F-fluoride PET/CT images were acquired in different scanners, the same software was employed in all quantifications, guaranteeing uniformity in the metrics. To our knowledge, this is the first study to evaluate the role of interim 18F-fluoride PET/CT in predicting the response to 223RaCl2 therapy, using quantitative methods to determine the skeletal tumor burden. It would be interesting to know whether these findings could be replicated in other populations, such as that of breast cancer patients treated with 223RaCl2. CONCLUSION In prostate cancer patients undergoing 223RaCl2 therapy, interim 18F-fluoride PET/CT performed after three cycles of 223RaCl2 does not seem able to predict outcomes. It also appears to be unable to differentiate a flare response from progressive disease, and we therefore discourage the use of interim 18F-fluoride PET/CT to evaluate the response to 223RaCl2 therapy in prostate cancer patients. There is a need for studies involving a larger number of patients and patients with other types of cancer, in order to verify our findings. Acknowledgments We would like to thank Patricia S. Fox for her advice regarding the statistical analysis. Financial support This work received funding from the James E. Anderson Distinguished Professorship Endowment and from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation). REFERENCES 1. Etchebehere EC, Araujo JC, Fox PS, et al. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56:1177–84. 2. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213–23. 3. Kluetz PG, Pierce W, Maher VE, et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2014;20:9–14. 4. Joung JY, Ha YS, Kim IY. Radium Ra 223 dichloride in castration-resistant prostate cancer. Drugs Today (Barc). 2013;49:483–90. 5. Glendenning J, Cook G. Imaging breast cancer bone metastases: current status and future directions. Semin Nucl Med. 2013;43:317–23. 6. Azad GK, Cook GJ. Multi-technique imaging of bone metastases: spotlight on PET-CT. Clin Radiol. 2016;71:620–31. 7. Bacher U, Binder M. Modifying therapy in patients with advanced Hodgkin’s lymphoma by integrating early metabolic response by interim PET-CT. Ann Transl Med. 2016;4(Suppl 1):S19. 8. Koo PJ, Kim SJ, Chang S, et al. Interim fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography to predict pathologic response to preoperative chemoradiotherapy and prognosis in patients with locally advanced rectal cancer. Clin Colorectal Cancer. 2016;15:e213–9. 9. Pahk K, Rhee S, Cho J, et al. The role of interim 18F-FDG PET/CT in predicting early response to neoadjuvant chemotherapy in breast cancer. Anticancer Res. 2014;34:4447–55. 10. Chen SW, Hsieh TC, Yen KY, et al. Interim FDG PET/CT for predicting the outcome in patients with head and neck cancer. Laryngoscope. 2014;124:2732–8. 11. Joensuu T, Joensuu G, Kairemo K, et al. Multimodal primary treatment of metastatic prostate cancer with androgen deprivation and radiation. Anticancer Res. 2016;36:6439–47. 12. Rohren EM, Etchebehere EC, Araujo JC, et al. Determination of skeletal tumor burden on 18F-fluoride PET/CT. J Nucl Med. 2015;56:1507–12. 13. Nome R, Hernes E, Bogsrud TV, et al. Changes in prostate-specific antigen, markers of bone metabolism and bone scans after treatment with radium-223. Scand J Urol. 2015;49:211–7. 14. Brito AE, Santos A, Sasse AD, et al. 18F-fluoride PET/CT tumor burden quantification predicts survival in breast cancer. Oncotarget. 2017;8:36001–36011. 15. Wade AA, Scott JA, Kuter I, et al. Flare response in 18F-fluoride ion PET bone scanning. AJR Am J Roentgenol. 2006;186:1783–6. 16. Kurdziel KA, Shih JH, Apolo AB, et al. The kinetics and reproducibility of 18F-sodium fluoride for oncology using current PET camera technology. J Nucl Med. 2012;53:1175–84. 17. Apolo AB, Lindenberg L, Shih JH, et al. Prospective study evaluating Na18F PET/CT in predicting clinical outcomes and survival in advanced prostate cancer. J Nucl Med. 2016;57:886–92. 18. Yu EY, Duan F, Muzi M, et al. Castration-resistant prostate cancer bone metastasis response measured by 18F-fluoride PET after treatment with dasatinib and correlation with progression-free survival: results from American College of Radiology Imaging Network 6687. J Nucl Med. 2015;56:354–60. 19. Cook G Jr, Parker C, Chua S, et al. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223 Ra-chloride (Alpharadin). EJNMMI Res. 2011;1:4. 20. Messiou C, Cook G, de Souza NM. Imaging metastatic bone disease from carcinoma of the prostate. Br J Cancer. 2009;101:1225–32. 21. Pollen JJ, Witztum KF, Ashburn WL. The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. AJR Am J Roentgenol. 1984;142:773–6. 22. Kairemo K, Joensuu T. Radium-223-dichloride in castration resistant metastatic prostate cancer—preliminary results of the response evaluation using F-18-fluoride PET/CT. Diagnostics (Basel). 2015;5:413–27. 1. Universidade Estadual de Campinas (Unicamp), Campinas, SP, Brazil; a. https://orcid.org/0000-0002-8632-5943 2. Real Hospital Português de Beneficência em Pernambuco – Real Nuclear, Recife, PE, Brazil; b. https://orcid.org/0000-0002-1065-9672 3. The University of Texas MD Anderson Cancer Center, Houston, TX, USA; c. https://orcid.org/0000-0002-5194-746X 4. The University of Texas MD Anderson Cancer Center, Houston, TX, USA; d. https://orcid.org/0000-0002-9788-6471 5. The University of Texas MD Anderson Cancer Center, Houston, TX, USA 6. The University of Texas MD Anderson Cancer Center, Houston, TX, USA Correspondence: Dra. Ana Emília Brito Real Hospital Português de Beneficência em Pernambuco – Real Nuclear Avenida Governador Agamenon Magalhães, 4760, Derby Recife, PE, Brazil, 52010-902 Email: aetbrito@gmail.com Received 3 October 2017 Accepted after revision 23 January 2018 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554