Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 51 nº 6 - Nov. / Dec. of 2018
Vol. 51 nº 6 - Nov. / Dec. of 2018
|
ORIGINAL ARTICLES
|
|
Pulmonary inhalation-perfusion scintigraphy in the evaluation of bronchoscopic treatment of bronchopleural fistula |
|
|
Autho(rs): Carla Rachel Ono1,a; Miguel Lia Tedde2,b; Paulo Rogerio Scordamaglio3,c; Carlos Alberto Buchpiguel4,d |
|
|
Keywords: Radionuclide imaging/methods; Radioactive tracers; Bronchial fistula; Septal occluder device; Lung. |
|
|
Abstract: INTRODUCTION
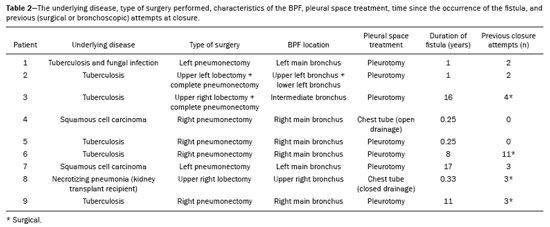
A bronchopleural fistula (BPF) has been defined as a direct communication between a bronchus and the pleural space. A peripheral BPF is a fistulous communication between the airway distal to the segmental bronchi or lung parenchyma and the pleura, occurring after infection, rheumatic diseases, necrotizing pneumonia, empyema, radiotherapy, bulla rupture, or interventional procedures. A central BPF is a fistulous communication between the trachea or segmental bronchi and the pleura, occurring after lung resection or traumatic disruption of the tracheobronchial tree. Although rare, a BPF is one of the most serious life-threatening complications of pulmonary resection(1,2). Dehiscence of the bronchial stump after pulmonary resection continues to be the most common cause of BPF. The reported incidence of BPF ranges from 0.5% to 3.0% after lobectomy and from 2% to 20% after pneumonectomy, its occurrence typically being associated with high morbidity and mortality. In patients with BPF, the air leak through the fistula makes the situation more dramatic because it impairs ventilation and phonation, as well as increasing pleural space secretions(3,4). Bronchoscopy is one of the most accurate methods to identify a BPF, which is often challenging, especially when the BPF is small(5). A crucial step in the treatment of patients with BPF involves drainage of the pleural space, which, by definition, is contaminated, the drainage protecting the contralateral lung from leakage of pleural fluid via the BPF path. When possible, early central BPFs should be treated surgically, through repair of the bronchial stump. Although surgical correction is the treatment of choice, some patients are not suitable candidates for another surgical resection. In this scenario, a variety of minimally invasive transbronchial methods, including the use of occlusive agents (e.g., fibrin sealants), coils, stents, or one-way valves, have been employed in order to close the central BPF directly(6-10). Such bronchoscopic treatments are successful only when the BPF is small. For larger fistulas, such as those caused by complete dehiscence of the stump (the so-called "total" fistulas), none of the endoscopic treatments have proven effective. In the present study, we opted to treat patients with total BPF through bronchoscopic placement of occluders originally developed for percutaneous closure of cardiac septal defects(11). After the treatment, we followed those patients by performing periodic bronchoscopic evaluations. However, the presence of the occluder within the bronchial stump decreases the accuracy of such evaluations. The purpose of this study was to evaluate the value and usefulness of nuclear pulmonary inhalation-perfusion scintigraphy as an alternative method of investigation and follow-up of patients with BPFs. MATERIALS AND METHODS Study design and patient sample This was a prospective study evaluating the safety and efficacy of endoscopic treatment of total BPFs through the off-label use of a transcatheter atrial septal defect occluder (Figulla ASD N; International Occlutech AB, Helsingborg, Sweden), as depicted in Figure 1, and the use of a pulmonary inhalation-perfusion scintigraphy as a method of detecting a residual BPF. The study was approved by the Committee for the Analysis of Research Projects of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), reference no. 1089/09, and was registered with ClinicalTrials.gov (identifier: NCT01153074; http://www.clinicaltrials.gov/). 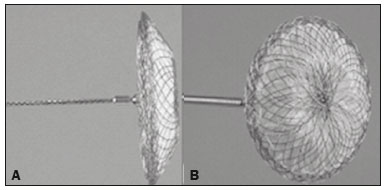 Figure 1. The Occlutech Figulla ASD N device. A: Lateral view. B: Frontal view. The study design included an initial bronchoscopic examination to measure the BPF diameter, assess the degree of mucosal inflammation along the fistula trajectory, and obtain biopsy samples to exclude the presence of residual disease. Inhalation-perfusion scintigraphy was performed at baseline (before the occluder was put in place). Patients were followed through periodic bronchoscopy. A final evaluation, conducted at 12 months after the bronchoscopic occlusion of the fistulas, included a complete clinical interview (to check for residual clinical symptoms such as dyspnea and difficulty in phonation) and a check for air leaks through the thoracostomy or chest tube, as well as bronchoscopy with a methylene blue test and another inhalation-perfusion scintigraphy examination. The study sample comprised nine patients with total BPFs, all of whom had undergone open drainage of the pleural space by pleurotomy or chest tube and were not suitable candidates for surgical correction. The demographic, anthropometric, and clinical characteristics of the patients, including the Medical Research Council dyspnea score(12), are shown in Table 1. 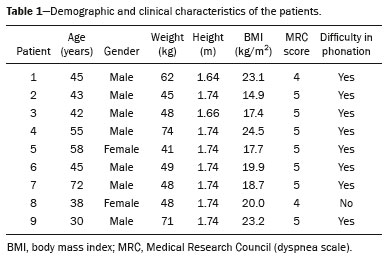 The underlying disease, the type of surgery performed, the location of the BPF, the treatment of the ipsilateral pleural space, the time since the initial appearance of the fistula, and the number of previous (surgical or bronchoscopic) attempts at closure of the fistula are described in Table 2. The median time from the BPF occurrence to treatment was 5.93 years (range, 0.25-17.0 years), and there had been previous (surgical or bronchoscopic) attempts at closure of the fistula in all of patients except patients 3 and 4. After the first bronchoscopic evaluation, the patients were submitted to a baseline pulmonary inhalation-perfusion scintigraphy. The inclusion criterion was having a BPF detected by scintigraphy. The technique utilized to place the occluders has been previously described in detail(13). Pulmonary inhalation-perfusion scintigraphy All scintigraphy procedures were performed in the Nuclear Medicine Division of the HC-FMUSP Department of Radiology, using a dual-head gamma camera (E-Cam; Siemens Medical Solutions, Chicago, IL, USA). Prior to inhalation of the radiopharmaceutical and the acquisition of images, the thoracostomy was occluded with a bandage or the chest tube was closed. Each patient inhaled, via a nebulizer, 900-1300 MBq (25-35 mCi) of technetium-99m-labeled diethylenetriaminepentaacetic acid (99mTc-DTPA). All patients were kept in the upright position, and, during tidal breathing with the nose occluded, the aerosolized 99mTc-DTPA was administered through a mouthpiece over a period of five minutes. The estimated level of activity that was reached and maintained in the lungs was 20-40 MBq (0.5-1.0 mCi). Image acquisition After the inhalation of the radiopharmaceutical, planar images of the chest were obtained in anterior, posterior, lateral, and oblique views. With a 128 × 128 matrix and a low-energy, high-resolution collimator, each planar image accumulated 500 K counts. The system was calibrated for an energy photopeak of 140 keV with a 15% window. One additional image was acquired in the anterior view of the chest with a flood source behind the patient in order to delineate the contours of the body. Perfusion scintigraphy The perfusion scintigraphy scans were obtained after the inhalation scintigraphy scans. The perfusion scans were acquired after intravenous administration of 185 MBq (5 mCi) of 99mTc-labeled macroaggregated albumin (99mTc-MAA). Imaging acquisition Planar images of the chest were obtained in anterior, posterior, lateral, and oblique views. With a 128 × 128 matrix and a low-energy, high-resolution collimator, each planar image accumulated 1500 K counts. The system was calibrated for an energy photopeak of 140 keV with a 15% window. Follow-up and statistical analysis We have provided two examples of scintigraphy examinations. One shows the 99mTc-DTPA activity in the bandage occluding the wound on the left side of the chest wall, revealing the BPF (Figure 2). The other example demonstrates no signs of a BPF in a patient submitted to right pneumonectomy (Figure 3). 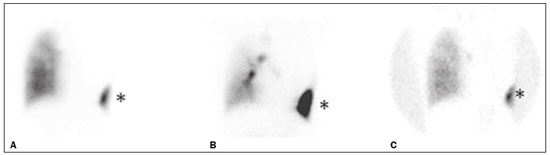 Figure 2. Patient submitted to left pneumonectomy and inhalation-perfusion scintigraphy. A: Scintigraphy with 99mTc-MAA inhalation, anterior view in the perfusion scan. B: Scintigraphy with 99mTc-DTPA inhalation, anterior view in the ventilation scan. C: Scintigraphy with 99mTc-DTPA inhalation, anterior view and a silhouette contour in the inhalation scan. The images demonstrated no radiopharmaceutical activity in the left lung. Note the intense radiopharmaceutical activity at the bandage occluding the wound (asterisk) on the left side of the chest wall, demonstrating the BPF, in the inhalation scan. In the perfusion scan (A), residual radiopharmaceutical activity from the inhalation scan can be seen. 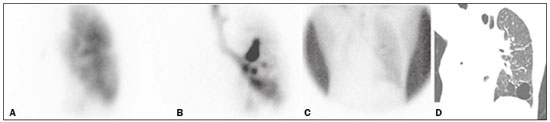 Figure 3. Patient submitted to right pneumonectomy and inhalation-perfusion scintigraphy. A: Scintigraphy with 99mTc-MAA inhalation, anterior view in the perfusion scan. B: Scintigraphy with 99mTc-DTPA inhalation, anterior view in the ventilation scan. C: Scintigraphy with 99mTc-DTPA inhalation, anterior view and a silhouette contour in the ventilation scan. D: Coronal computed tomography scan, with a lung window setting. The images demonstrated no radiopharmaceutical activity in the right lung. There were no signs of BPF. As previously mentioned, bronchoscopy was repeated at 12 months after placement of the occluder, in order to evaluate the effectiveness of the treatment (Figure 4). The possibility of residual fistula was explored through instillation of methylene blue into the treated bronchial stump. The visualization of the dye in the pleural drain or pleurotomy was considered a strong indicator of residual air leak. Another ventilation scan was also acquired at that time. 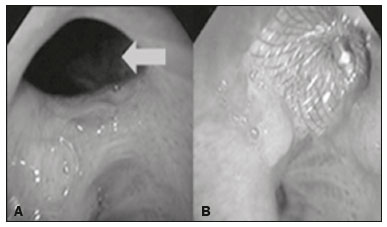 Figure 4. Bronchoscopic view of a BPF in the upper right bronchus: A: Pretreatment. B: At 12 months after placement of the occluder in the fistula trajectory. A favorable outcome was defined as complete closure of the fistula, as confirmed by bronchoscopy, together with a negative methylene blue test or significant improvement of symptoms and the device being well positioned in the stump. Continuous variables are expressed as means and standard deviations, whereas categorical variables are expressed as absolute and relative frequencies. RESULTS Most (66.7%) of the BPFs evaluated were on the right side, and the mean diameter was 15 ± 3.65 mm (range, 6-17 mm). The initial bronchoscopic evaluation showed that all fistulas presented well-defined edges, suggesting a chronic process, and the biopsy of the mucosa along the fistula trajectory excluded active local disease in all cases. The pulmonary inhalation-perfusion scintigraphy obtained at baseline confirmed the presence of the radioactive tracers in the thoracostomy bandage in all nine patients (Figure 2). In two patients (cases 2 and 4) the occluder was withdrawn: in one, because a wire broke during an attempt to reposition the device; in the other, because the patient was severely undernourished and had not developed granulation tissue after one year. Two other patients (corresponding to cases 5 and 6) died from unrelated causes, which precluded their inclusion in the final evaluation. Therefore, the final sample comprised five patients. The results of the baseline pulmonary inhalation-perfusion scintigraphy, together with aspects observed at 12 months after placement of the occluder, including the clinical symptoms, the bronchoscopic aspect of the bronchial stump, the methylene blue test results, and the results of the final scintigraphy are presented in Table 3. 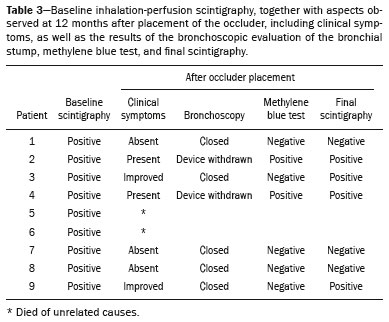 The placement of the device in the bronchial stump promoted a significant improvement of clinical symptoms in the majority of the patients. In most cases, the patients showed improved respiration and phonation, as well as a reduction in pleural secretions and an improvement in their nutritional status. The improvement in clinical symptoms persisted while the device remained in the stump. In two cases, there was a residual air leak that was not identified by bronchoscopy or the methylene blue test but was detected only by pulmonary inhalation-perfusion scintigraphy. Those results correlated with the evolution of the patients, both of whom showed late signs of air leak, confirming the scintigraphy findings. DISCUSSION A BPF and the subsequent pleural space contamination constitute one of the most serious postoperative complications after pulmonary resection. If not drained, the massive secretions from the pleural space can be aspirated through the fistula, choking the patient or contaminating the contralateral lung. That is why all of the patients in our sample had a chest tube or a pleurotomy. However, drainage of the pleural space creates a route for a major air leak that can hamper respiration and phonation. The rational for bronchoscopic treatment of BPF in patients whose clinical condition precludes surgical correction is the fact that the placement of an occluder in the bronchial stump results in rapid improvement of symptoms. In a previous study conducted by our group, we reported our experience with the bronchoscopic treatment of fistulas(14-16). A BPF can be detected by several imaging modalities other than bronchoscopy, including chest X-ray and multidetector computed tomography employing advanced image post-processing techniques. However, the presence of the occluder within the bronchial stump, in the BPF trajectory, could produce an artifact in the above mentioned radiographic methods and could preclude better evaluation through bronchoscopy. That is the rationale for choosing lung inhalation scintigraphy as an alternative imaging method to evaluate the BPF before and after endoscopic treatment. Greyson et al.(17) were the first to demonstrate that scintigraphy with inhalation of a radionuclide (99mTc-albumin colloid fog) is a simple and accurate test for the detection of a BPF. In addition to 99mTc-albumin colloid fog, a variety of radioactive tracers, including 99mTc-sulfur colloid, 99mTc-DTPA, or a gas like 133Xe, could be alternative radiopharmaceuticals for use in inhalation-perfusion scintigraphy(18,19). Mark et al.(20) reported their experience using inhalation-perfusion scintigraphy with 99mTc-DTPA inhalation in 28 patients who had undergone pneumonectomy, showing that, for the detection of a BPF, the method had a sensitivity of 78%, a specificity of 100%, and an accuracy of 86%. Nevertheless, it lacks accuracy in the detection of very small BPFs, is time-consuming, and requires patient cooperation, which can be difficult during the postoperative period, when the patients could be on mechanical ventilation, could be critically ill, or could have sepsis. Pulmonary inhalation-perfusion scintigraphy has other known limitations. The turbulent flow in the tracheobronchial tree, which promotes aerosol deposition, can lead to false-positive results in patients with chronic obstructive pulmonary disease. Therefore, this modality is currently used only when conventional bronchoscopy, virtual bronchoscopy, and multidetector computed tomography have all failed to identify a clinically suspected BPF(21). Although there is no standardization of pulmonary inhalation-perfusion scintigraphy for monitoring these cases, the technique used in the present study provided excellent results that were strongly correlated with clinical improvement and bronchoscopic findings, even in long-term clinical follow-up. In the patients with complete resolution of symptoms, closure of the fistula confirmed by bronchoscopy, and no evidence of dye leakage, the inhalation-perfusion scintigraphy was completely negative. In cases of failure to close the BPF, the scintigraphy confirmed the persistence of the air leak. In two (40%) of the five patients in our sample, scintigraphy was the only method to show residual BPF, which was not detected in the bronchoscopic assessment or in the methylene blue test (no dye extravasation into the pleural space). Therefore, to avoid events related to the severe sepsis that could occur if the space closed prematurely, the thoracostomy was maintained, thus minimizing the risk, in those two patients. In the literature, 99mTc-DTPA aerosol inhalation scintigraphy has been reported to have poor sensitivity. However, in the present study, the initial inhalation-perfusion scintigraphy detected BPF in all of the patients evaluated. That is probably because open drainage of the hemithorax (with thoracostomy or a chest tube) was employed in all of the patients in our sample. Such drainage likely promotes the flow directly out of the thoracic cavity, as well as allowing the detection of radioactive activity in the occlusive bandage or adjacent to the chest wall (on its outer face). The detection of radiopharmaceutical activity in the bandage or in the additional image obtained with the flood source facilitated the localization of such activity outside of the body(22). Our results shows that pulmonary inhalation-perfusion scintigraphy is a useful tool to identify residual BPFs, even when classical methods such as bronchoscopy and the blue methylene test fail to detect it. Additional studies with larger patient samples are needed in order to confirm our preliminary findings. REFERENCES 1. Deschamps C, Bernard A, Nichols FC 3rd, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg. 2001;72:243-7. 2. Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg. 2001;7:330-6. 3. Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg. 2001;13:3-7. 4. Turk AE, Karanas YL, Cannon W, et al. Staged closure of complicated bronchopleural fistulas. Ann Plast Surg. 2000;45:560-4. 5. Seo H, Kim TJ, Jin KN, et al. Multi-detector row computed tomographic evaluation of bronchopleural fistula: correlation with clinical, bronchoscopic, and surgical findings. J Comput Assist Tomogr. 2010;34:13-8. 6. Gonfiotti A, Santini PF, Jaus M, et al. Safety and effectiveness of a new fibrin pleural air leak sealant: a multicenter, controlled, prospective, parallel-group, randomized clinical trial. Ann Thorac Surg. 2011;92:1217-24. 7. Sivrikoz CM, Kaya T, Tulay CM, et al. Effective approach for the treatment of bronchopleural fistula: application of endovascular metallic ring-shaped coil in combination with fibrin glue. Ann Thorac Surg. 2007;83:2199-201. 8. Tanaka S, Yajima T, Mogi A, et al. Successful management of a large bronchopleural fistula after lobectomy: report of a case. Surg Today. 2011;41:1661-4. 9. Walsh MD, Bruno AD, Onaitis MW, et al. The role of intrathoracic free flaps for chronic empyema. Ann Thorac Surg. 2011;91:865-8. 10. Wu G, Li ZM, Han XW, et al. Right bronchopleural fistula treated with a novel, Y-shaped, single-plugged, covered, metallic airway stent. Acta Radiol. 2013;54:656-60. 11. Scordamaglio PR, Tedde ML, Minamoto H, et al. Can total bronchopleural fistulas from complete stump dehiscence be endoscopically treated? Eur J Cardiothorac Surg. 2017;51:702-8. 12. Fletcher CM, Clifton M, Fairbairn AS, et al. Standardized questionaries on respiratory symptoms. Br Med J. 1960;2:1665. 13. Tedde ML, Scordamaglio PR, Minamoto H, et al. Endobronchial closure of total bronchopleural fistula with Occlutech Figulla ASD N device. Ann Thorac Surg. 2009;88:e25-6. 14. Scordamaglio PR, Tedde ML, Minamoto H, et al. Endoscopic treatment of tracheobronchial tree fistulas using atrial septal defect occluders: preliminary results. J Bras Pneumol. 2009;35:1156-60. 15. Tedde ML, Minamoto H, Scordamaglio PR, et al. Broncoscopic closure of tracheoesophageal fistulas. Ann Thorac Surg. 2011;91:1311. 16. Tedde ML, Scordamaglio PR, Rodrigues A, et al. Minimally invasive closure of bronchopleural fistulas. Chest. 2011;140:826. 17. Greyson ND, Rosenthal L. Detection of postoperative bronchopleural fistulas by radionuclide fog inhalation. Can Med Assoc J. 1970;103:1366-8. 18. Nielsen KR, Blake LM, Mark JB, et al. Localization of bronchopleural fistula using ventilation scintigraphy. J Nucl Med. 1994;35:867-9. 19. Pigula FD, Keenan RJ, Naunheim KS, et al. Diagnosis of postpneumonectomy bronchopleural fistula using ventilation scintigraphy. Ann Thorac Surg. 1995;60:1812-4. 20. Mark JB, McDougall IR. Diagnosis and localization of bronchopulmonary air leaks using ventilation scintigraphy. Chest. 1997;111:286-9. 21. Gaur P, Dunne R, Colson YL, et al. Bronchopleural fistula and the role of contemporary imaging. J Thorac Cardiovasc Surg. 2014;148:341-7. 22. Raja S, Rice TW, Neumann DR, et al. Scintigraphic detection of post-pneumonectomy bronchopleural fistulae. Eur J Nucl Med. 1999;26:215-9. 1. Nuclear Medicine Division, Instituto de Radiologia do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil; a http://orcid.org/0000-0002-0179-7248 2. Department of Thoracic Surgery, Instituto do Coração do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InCor/HC-FMUSP), São Paulo, SP, Brazil; b http://orcid.org/0000-0002-8178-4243 3. Respiratory Endoscopy Division, Instituto do Coração do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InCor/HC-FMUSP), São Paulo, SP, Brazil; c http://orcid.org/0000-0001-8971-5333 4. Nuclear Medicine Division, Instituto de Radiologia do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil; d http://orcid.org/0000-0003-0956-2790 Correspondence: Dr. Miguel L. Tedde Rua Itambé, 367, ap. 151-A, Higienópolis São Paulo, SP, Brazil, 01239-001 E-mail: tedde@usp.br Received August 09, 2017 Accepted after revision November 03, 2017 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554