Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 50 nº 6 - Nov. / Dec. of 2017
Vol. 50 nº 6 - Nov. / Dec. of 2017
|
ORIGINAL ARTICLE
|
|
SPECT/CT with radiolabeled somatostatin analogues in the evaluation of systemic granulomatous infections |
|
|
Autho(rs): Paulo Henrique Silva Monteiro1; Thiago Ferreira de Souza1; Maria Luiza Moretti2; Mariangela Ribeiro Resende2; Jair Mengatti3; Mariana da Cunha Lopes de Lima1; Allan Oliveira Santos1; Celso Darío Ramos1 |
|
|
Keywords: Single photon emission computed tomography; X-ray computed tomography; Octreotide; Gallium-67; Tuberculosis; Paracoccidioidomycosis. |
|
|
Abstract: INTRODUCTION
Octreotide is a somatostatin analogue with several clinical applications, such as controlling upper gastrointestinal bleeding and treating carcinoid syndrome(1). When radiolabeled, it becomes an important diagnostic and therapeutic tool for neuroendocrine tumors. It was initially labeled with indium-111 (111In), as 111In-DTPA-octreotide(2). Subsequently, tyrosine-octreotide radiolabeled with metastable technetium-99 (99mTc-EDDA-HYNIC-TOC) became available and was shown to have several advantages over 111In-DTPA-octreotide, including lower cost, ease of storage in a freeze-dried kit for labeling with 99mTc, better image quality, and a faster image acquisition protocol(3,4). When activated, monocytes and lymphocytes show strong membrane expression of somatostatin receptors(5,6). Therefore, we expected to detect marked expression of those receptors, measurable by radiolabeled somatostatin analogue (RSA) scintigraphy, in granulomas and activated leukocytes that manifested as an immune response to systemic infectious diseases, such as tuberculosis, paracoccidioidomycosis, histoplasmosis, opportunist infections, pneumocystosis, and aspergillosis(7). Those diseases, as a group, have a high prevalence in many developing countries and a high number of new cases per year. Owing to the nature of their physiology, these diseases tend to spread throughout the whole body. The treatment is usually slow and often empirical, especially because there are only a few reliable methods to evaluate the disease activity before, during, and after treatment(8–10). Conventional computed tomography (CT) can produce false-negative results for disease activity during and after treatment(11,12). Gallium-67 (67Ga) scintigraphy has long been used in order to detect and evaluate systemic granulomatous infections and is in fact considered as the gold standard for evaluating such infections(13,14). However, it is difficult to use 67Ga in many situations, because it must be ordered days ahead of the moment of the procedure and, in many countries, only on specific days of the week. In addition, in specific situations, it is necessary to acquire images at 48–72 h after injection, which limits its use in urgent and acute care. The objectives of this study were to evaluate RSA uptake in patients with systemic granulomatous infections and to compare it with 67Ga citrate uptake, which was used as the reference method. MATERIALS AND METHODS This was an observational, prospective study. The following inclusion criteria were applied: having a confirmed diagnosis, either clinically or according to laboratory test results, of a systemic infectious disease (such as tuberculosis, paracoccidioidomycosis, histoplasmosis, opportunistic infections, pneumocystosis, and aspergillosis); having been referred to our facility for 67Ga citrate scintigraphy; being under regular inpatient or outpatient follow-up of infectious diseases; and being 18 years of age or older. Patients who were pregnant or breastfeeding were excluded, as were those who declined to submit to or did not tolerate either of the imaging procedures at any time. All procedures were performed in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participating patients gave written informed consent. We studied 28 consecutive patients (11 females), with a mean age of 43.2 ± 14.9 years (range, 18–76 years), all of whom had active systemic granulomatous infection: tuberculosis (in 13); paracoccidioidomycosis (in 8); pneumocystosis (in 2); cryptococcosis (in 1); angioinvasive pulmonary aspergillosis (in 1); leishmaniasis (in 1); Bartonella henselae infection-associated vasculitis (in 1); and an unspecified opportunistic infection, treated empirically as tuberculosis (in 1). Of the 28 patients, 23 had already started specific treatment for the diverse infectious diseases an average of 19 ± 23 days before their inclusion in the study. The aspergillosis, cryptococcosis, and pneumocystosis patients were immunocompromised. Almost all of the patients had lung disease, except for the patient with B. henselae infection-associated vasculitis, who had diffuse large-vessel disease. All patients were assessed through clinical interviews and examinations, as well as through imaging studies with 99mTc-EDDA-HYNIC-TOC or 111In-DTPA-octreotide, depending on radiopharmaceutical availability. All patients were also submitted to 67Ga citrate imaging, except for one patient who died before the radiotracer became available. In that patient, the 99mTc-EDDA-HYNIC-TOC imaging findings were correlated with those of a guided biopsy. The interval between imaging sessions did not exceed 10 days (range, 4–10 days). For all patients, current diseases, known disease activity foci, disease duration, disease progress, disease severity, and responses to previous therapies were evaluated in the clinical interviews and by reviewing patient charts. Scintigraphy with 99mTc-EDDA-HYNIC-TOC was performed in a SPECT/CT system (Symbia T2; Siemens, Erlangen, Germany) with a low-energy, high-resolution collimator. The radiotracer dose was 249–370 MBq, corrected for patient body weight, with a mean dose of 300 MBq. In all patients, whole-body SPECT/CT scanning (table speed, 6 cm/min) was performed at 4 h after injection and was followed by additional SPECT/CT scans (matrix, 64 × 64 pixels; 360° detector configuration, with a rotation of 180° for each detector; 6° per step; 30 s per view; and a 2-slice CT with voltage and current set at 130 kV and 35 mA, respectively) of regions of interest selected by an analysis of the whole-body scan. In some patients, additional planar images (1,000,000 counts per static image) were acquired 24 h after radiotracer injection, when it was deemed necessary in order to clarify inconclusive imaging findings or to resolve discrepancies between the clinical data and the images. Scintigraphy with 111In-DTPA-octreotide was performed in the same SPECT/CT system described above, with a high-energy collimator and a radiotracer dose of 185 MBq. In all patients, whole-body SPECT/CT scanning (table speed, 3 cm/min) was performed 24 h after injection and was followed by additional SPECT/CT scans (matrix, 64 × 64 pixels; 360° detector configuration, with a rotation of 180° for each detector; 6° per step; 35 s per view; and a 2-slice CT with voltage and current set at 130 kV and 35 mA, respectively) of regions of interest selected by an analysis of the whole-body scan. As previously stated, all but one of the patients underwent 67Ga scintigraphy. The 67Ga scintigraphy was performed in the same SPECT/CT system described above, with a high-energy collimator and a radiotracer dose of 185 MBq. In all patients, whole-body SPECT/CT scanning (table speed, 8 cm/min) was performed 48 h after injection and was followed by additional SPECT/CT scans (matrix, 64 × 64 pixels; 360° detector configuration, with a rotation of 180° for each detector; 6° per step; 30 s per view; and a 2-slice CT with voltage and current set at 130 kV and 35 mA, respectively) of regions of interest, selected by an analysis of the whole-body scan. RESULTS Twenty-seven sites of focal or diffuse active infectious disease were detected by 67Ga in 20 of the 27 patients submitted to 67Ga scintigraphy. All of those sites were also detected in RSA images (Figure 1). The images obtained with both tracers were negative in the other 7 patients. The intensity of 67Ga uptake was visually mild in 5 patients, moderate in 11, and marked in 4. The intensities of 99mTc-EDDA-HYNIC-TOC and 111In-DTPA-octreotide uptake in the lesions were visually mild in 13 patients, moderate in 5, and marked in 2. The intensity of RSA uptake was visually lower than 67Ga uptake in 11 of the 20 patients with positive images (Figure 2), which was best visualized in SPECT/CT. In the 9 remaining patients, the RSA uptake in the sites of active disease was similar to 67Ga uptake. The only patient who did not undergo 67Ga scintigraphy was submitted to 99mTc-EDDA-HYNIC-TOC SPECT/CT-guided biopsy of a right-lung cavity with moderate focal uptake. That patient had multiple lung cavities and was under suspicion of having an undiagnosed opportunistic infection, dying before 67Ga was available for injection. The lung biopsy revealed aspergillosis (Figure 3). 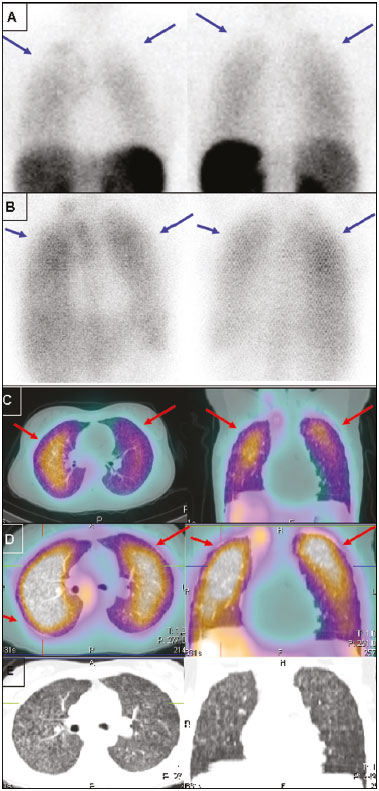 Figure 1. Static lung images (A,B) and transaxial SPECT/CT images (C,D) with 99mTc-EDDA-HYNIC-TOC (A,C) and 67Ga citrate (B,D) of an 18-year-old patient with miliary tuberculosis, plus the CT imaging (E) performed for the scan (2-slice CT, obtained during respiration to avoid mismatching artifacts with the SPECT image). Note the diffuse lung uptake, a pattern typical of miliary tuberculosis, detected on both exams (blue arrows on static images, red arrows on SPECT/CT imaging). 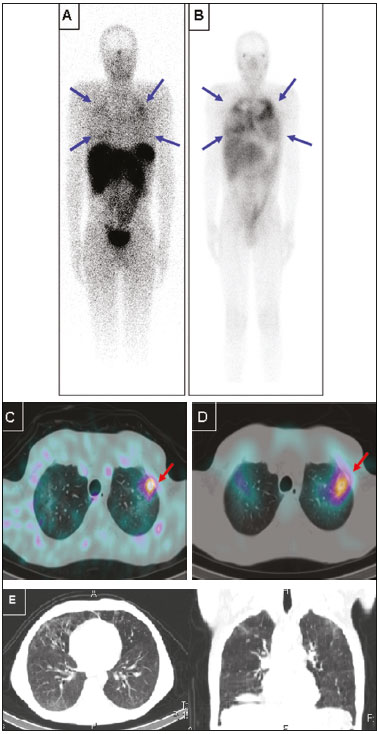 Figure 2. Anterior whole-body images (A,B) and transaxial SPECT/CT images (C,D) with 99mTc-EDDA-HYNIC-TOC (A,C) and 67Ga citrate (B,D) of a 39-year-old patient with pneumocystosis. Note the heterogeneous increased uptake in both lungs, more intense on 67Ga citrate images (blue arrows). A focal area of more active disease in the left upper lobe is evident with both tracers, and is more clearly seen with SPECT/CT images (red arrows). Selected axial and coronal CT images are also displayed on (E), showing multiple areas of groundglass opacities. 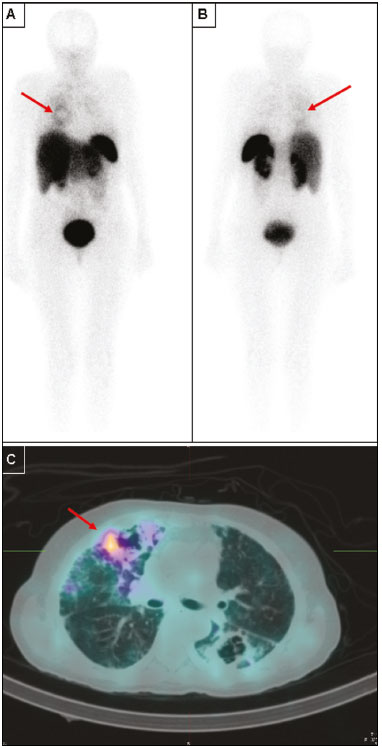 Figure 3. Anterior (A) and posterior (B) whole-body imaging and transaxial SPECT/CT image (C) with 99mTc-EDDA-HYNIC-TOC of a 51-year-old patient with stage C3 HIV infection (AIDS) and an undiagnosed opportunistic respiratory infection. After all serology studies, cultures, and usual examinations were negative for an etiology, it was decided to submit the patient to an unguided lung biopsy. On the day before the biopsy, she was brought to our attention, and 67Ga was unavailable in our service, prompting us to suggest 99mTc-EDDA-HYNIC-TOC imaging to guide the biopsy. Whole-body imaging, correlated with SPECT/CT, pointed to a single foci of more significant activity (red arrows), among several other inactive or barely active cavities, as seen on the SPECT/CT imaging. The biopsy was then guided to this cavity, and revealed Aspergillus sp., indicating angioinvasive pulmonary aspergillosis as the cause of the infection. However, due to the severity of the disease, the patient died before it was possible to perform 67Ga imaging. DISCUSSION For over 40 years, 67Ga citrate has been successfully used for the diagnosis and follow-up of diverse granulomatous diseases. However, its high cost, limited availability, and complicated imaging logistics limit its use at many nuclear medicine centers, especially centers in developing countries, which are precisely those at which the prevalence of infectious granulomatous diseases is highest. Therefore, there is a need for an alternative to 67Ga for the study of these diseases. In a pilot study, our group determined the potential of using RSA in that context(15). The results of the present study demonstrate concordance between RSA and 67Ga uptake sites in systemic granulomatous infections, suggesting that RSA scintigraphy could be used as an alternative method to detect disease activity and guide biopsies in the setting of clinically suspected granulomatous disease. Of particular interest is the case of the patient who did not manage to undergo 67Ga scintigraphy (Figure 3). That 51-year-old female patient presented a stage C3 HIV infection (AIDS) and several previous episodes of tuberculosis, which left her with multiple lung cavities. She was brought to our attention while suffering from a new, severe opportunistic infection, which did not respond to various empirical treatments, and her laboratory test results had not revealed the etiology of the infection. She was scheduled to undergo an unguided lung biopsy as a last-ditch effort to find the cause of the disease. She was included as part of our protocol, and her RSA imaging revealed moderate focal uptake in one specific cavity, in the right lung. The SPECT/CT image was then used to guide the lung biopsy, which identified the etiology as Aspergillus sp. Although the patient, due to disease progression, did not survive long enough for 67Ga to become available, we believe this case illustrates our intended purpose for this method: to quickly locate and direct the investigation of infectious granulomatous diseases. The method can detect which lesions identified by CT represent active infection, as opposed to residual scar tissue. That seems to be especially useful in patients with multiple prior treatments and suspected active infection, as well as in guiding further biopsies to the most active lesions. We also found limitations to the method investigated in the present study. The intensity of RSA uptake is less marked than is that of 67Ga and might require nuclear medicine practitioners who are more experienced in order to read the images. In addition, in our limited sample, RSA imaging did not reveal any additional lesions when compared with 67Ga, suggesting that the former is not superior in terms of sensitivity. On the contrary, RSA sensitivity is potentially inferior to that of 67Ga, because the lesions tended to uptake less RSA than 67Ga. Furthermore, the aforementioned previous treatment might have lowered the sensitivity of both imaging techniques, as might have the 4–10 day interval between the two procedures. However, because the treatment for granulomatous diseases usually takes weeks or months, we do not believe that this was a critical factor. The most severe limitation of our study is the small size of our sample, especially the limited number of patients with each individual disease. Further studies, with larger samples, are needed in order to gather stronger evidence to support the use of RSA scintigraphy for diagnosing these diseases in clinical practice. That is a worthwhile pursuit, given the wider availability, reduced cost, and faster imaging of RSA scintigraphy when compared with 67Ga citrate scintigraphy, which is still the current gold standard for the functional imaging of these diseases in many countries. CONCLUSIONS SPECT/CT with 99mTc-EDDA-HYNIC-TOC or 111In-DTPA-octreotide seems to be a good alternative to 67Ga citrate scintigraphy for the evaluation of patients with systemic granulomatous infections, especially for detecting which lesions found on CT represent active infection, as opposed to residual scar tissue. That seems to be especially useful in patients with multiple prior treatments and suspected active infection, as well as in guiding biopsies to the most active lesions. However, further studies are needed in order to gather more evidence to support its use in diagnosing such diseases in clinical practice. Acknowledgment We would like to acknowledge the Instituto de Pesquisas Energéticas e Nucleares (IPEN) (Nuclear and Energy Research Institute) for providing us with the quantity of 111In-DTPA-octreotide required for our research. REFERENCES 1. Dogliotti L, Tampellini M, Stivanello M, et al. The clinical management of neuroendocrine tumors with long-acting repeatable (LAR) octreotide: comparison with standard subcutaneous octreotide therapy. Ann Oncol. 2001;12 Suppl 2:S105–9. 2. Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–31. 3. Hubalewska-Dydejczyk A, Fröss-Baron K, Mikolajczak R, et al. 99mTc-EDDA/HYNIC-octreotate scintigraphy, an efficient method for the detection and staging of carcinoid tumours: results of 3 years' experience. Eur J Nucl Med Mol Imaging. 2006;33:1123–33. 4. Gabriel M, Decristoforo C, Donnemiller E, et al. An intrapatient comparison of 99mTc-EDDA/HYNIC-TOC with 111In-DTPA-octreotide for diagnosis of somatostatin receptor-expressing tumors. J Nucl Med. 2003;44:708–16. 5. Marko J, Lamba R, Miller F, et al. OctreoScan positive Crohn's disease mimicking an ileal carcinoid tumor. J Clin Gastroenterol. 2008;42:66–8. 6. Ferone D, Boschetti M, Resmini E, et al. Neuroendocrine-immune interactions: the role of cortistatin/somatostatin system. Ann N Y Acad Sci. 2006;1069:129–44. 7. Kwekkeboom DJ, Krenning EP, Kho GS, et al. Somatostatin receptor imaging in patients with sarcoidosis. Eur J Nucl Med. 1998;25:1284–92. 8. Santín M, Podzamczer D, Ricart I, et al. Utility of the gallium-67 citrate scan for the early diagnosis of tuberculosis in patients infected with the human immunodeficiency virus. Clin Infect Dis. 1995;20:652–6. 9. Ray S, Talukdar A, Kundu S, et al. Diagnosis and management of miliary tuberculosis: current state and future perspectives. Ther Clin Risk Manag. 2013;9:9–26. 10. Yamaga LY, Benard G, Hironaka FH, et al. The role of gallium-67 scan in defining the extent of disease in an endemic deep mycosis, paracoccidioidomycosis: a predominantly multifocal disease. Eur J Nucl Med Mol Imaging. 2003;30:888–94. 11. Nardini S, Schiavon F, Zuin R, et al. Has the role of radiology changed in the fight against pulmonary tuberculosis? Radiol Med. 1995;89:49–56. 12. Moon WK, Han MH, Chang KH, et al. CT and MR imaging of head and neck tuberculosis. Radiographics. 1997;17:391–402. 13. Palestro CJ, Goldsmith SJ. The role of gallium and labeled leukocyte scintigraphy in the AIDS patient. Q J Nucl Med. 1995;39:221–30. 14. Love C, Palestro CJ. Radionuclide imaging of inflammation and infection in the acute care setting. Semin Nucl Med. 2013;43:102–13. 15. Monteiro PHS, Souza TF, Stucchi RB, et al. SPECT/CT with 99mTc-EDDA-HYNIC-TOC and 111In-DTPA-octreotide for evaluation of systemic granulomatous infections. J Nucl Med. 2014;55 Suppl 1:abstract 1974. 1. Division of Nuclear Medicine, Department of Radiology, School of Medical Sciences, State University of Campinas (Unicamp), Campinas, SP, Brazil 2. Division of Infectology, Department of Internal Medicine, School of Medical Sciences, State University of Campinas (Unicamp), Campinas, SP, Brazil 3. Nuclear Energy and Research Institute (IPEN), São Paulo, SP, Brazil Study conducted in the Department of Radiology, School of Medical Sciences, State University of Campinas (Unicamp), Campinas, SP, Brazil. Mailing address: Dr. Paulo Henrique Silva Monteiro Avenida Moraes Salles, 1610, Centro Campinas, SP, Brazil, 13010-002 E-mail: paulohsm42@gmail.com Received April 26, 2016. Accepted after revision November 3, 2016. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554