Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 49 nº 2 - Mar. / Apr. of 2016
Vol. 49 nº 2 - Mar. / Apr. of 2016
|
ORIGINAL ARTICLE
|
|
Pulmonary 64-MDCT angiography with 50 mL of iodinated contrast material in an unselected patient population: a feasible protocol |
|
|
Autho(rs): Henrique Simão Trad1; Gustavo Santos Boasquevisque1; Tiago Rangon Giacometti2; Catherine Yang Trad1; Orlando Salomão Zoghbi Neto1; Clovis Simão Trad1 |
|
|
Keywords: Tomography, X-ray computed; Contrast media; Angiography; Pulmonary embolism. |
|
|
Abstract: INTRODUCTION
Pulmonary computed tomography angiography (PCTA) has long been in use and has become the gold standard for the evaluation of patients with suspected pulmonary embolism(1,2). In the beginning, when single-detector scans were used, it was difficult to detect peripheral emboli(3) and long scan times demanded large volumes of contrast material(4,5). Despite advances in multidetector computed tomography (MDCT) and increases in the speed of acquisition of large volumes of data(6,7), there has been no significant reduction in the volume of iodinated contrast material required for pulmonary angiography in routine practice. In fact, there is extensive data in the literature demonstrating that PCTA is more efficacious and better able to depict small vessels than are other diagnostic methods(8). Although authors have reported reducing the contrast volume to 30-40 mL(9,10), their studies were conducted in selected populations under special conditions. Following a more pragmatic approach, as discussed extensively in a recent study conducted by Hartmann et al.(7), we proposed a simple standard protocol with 50 mL of iodinated contrast material, in an unselected patient population, in which we tested vascular enhancement and image quality. MATERIALS AND METHODS Our study sample included 32 patients-25 females and 7 males, with a mean age of 53 years (range, 22-90 years)- suspected of having acute or chronic pulmonary thromboembolism, who were referred to our outpatient clinic between September 2012 and December 2013. The body mass index ranged from 19.0 kg/m2 to 41.8 kg/m2 (mean, 28.1 kg/m2). All patients underwent PCTA in a 64-slice MDCT (64-MDCT) scanner (LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA), with acquisition parameters of 1.25 mm digital collimation, 0.5 s tube rotation, pitch ratio of 1375:1, tube voltage of 100 kV, and automatic tube current modulation (SmartmA; GE Healthcare). Because of technical constraints, a tube voltage of 120 kV was used in three patients: one who was obese (135 kg); and two who could not raise their arms over their heads. We injected 50 mL of iodinated contrast material (iopamidol 612 mg/mL, 300 mg I/mL, Iopamiron 300; Bracco, Milan, Italy) into an antecubital venous access, at a preferred rate of 4.5 mL/s. In four patients, the rate of injection was reduced to 4.0 mL/s due to poor venous access. The total injection time ranged from 11.0 s (at 4.5 mL/s) to 12.5 s (at 4.0 mL/s). In all patients, the injection of contrast material was followed by the infusion of 30 mL of normal saline at the same injection rate. Bolus tracking was applied in the superior vena cava at the aortic arch level (Figure 1), with no established threshold or automatic trigger for acquisition. Alternatively, acquisition was triggered when the superior vena cava was seen to be completely filled with contrast material. The scan acquisition delay was 6-7 s, sufficient time for the complete intravascular buildup of material in the pulmonary arterial tree.  Figure 1. Bolus tracking control image with a fully filled superior vena cava. Two experienced radiologists assessed image quality, in terms of the capacity to identify thromboembolism and signs of pulmonary hypertension, as well as enhancement of subsegmental pulmonary arteries and the presence of superior vena cava streak artifacts. As can be seen in Figure 2, vascular enhancement was assessed in the pulmonary trunk; in the right and left main pulmonary arteries; and in the right and left inferior pulmonary arteries. For each image, the regions of interest (ROIs) encompassed most of the vascular lumen. The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated for every ROI, noise being defined as the standard deviation of each. The SNR was calculated by dividing the ROI signal by its noise. In addition, in the pectoral muscles and the deep paraspinal muscles, at least four ROIs were measured bilaterally and averaged, the result being considered representative of the muscle signal. The CNR was calculated by subtracting the vascular signal from the muscle signal and dividing the result by the noise. 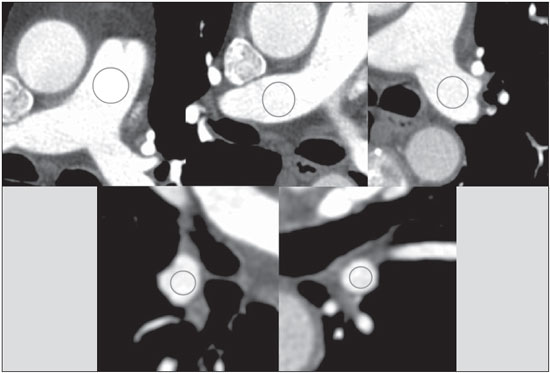 Figure 2. Assessment of vascular enhancement in the pulmonary trunk; right and left main pulmonary arteries; and right and left inferior pulmonary arteries. RESULTS In our study sample, the total scan time ranged from 2.2 to 2.8 s, with an mean of 2.5 s. The overall mean arterial density, in Hounsfield units (HU), was 384 HU (range, 189-634 HU). Specifically, the mean ± standard deviation was 395 ± 99 HU for the pulmonary trunk; 391 ± 94 HU and 390 ± 91 HU for the right and left main pulmonary arteries, respectively; and 315 ± 96 HU and 378 ± 91 HU for the right and left inferior pulmonary arteries, respectively. The overall mean SNR and CNR were 28.7 and 25.2, respectively, for the right main pulmonary arteries; 19.0 and 16.6, respectively, for the left main pulmonary arteries; 23.1 and 20.2, respectively, for the right inferior pulmonary arteries; and 26.1 and 22.7, respectively, for the left inferior pulmonary arteries. Subsegmental arteries were analyzed in all patients. In 8 patients, we identified pulmonary thromboembolism, which involved major branches in 3 and segmental to subsegmental arteries in 5. Signs of pulmonary hypertension were seen in 5 patients, all of whom showed enlarged pulmonary arteries, clear hypertrophy of the right ventricle being observed in only one case. However, among the patients with signs of pulmonary hypertension, only 2 showed thromboembolism in the current PCTA. Streak artifacts from residual contrast material in the superior vena cava were present in all patients, although it did not impede image analysis of the pulmonary arterial tree in any cases. The mean dose length product was 362.1 mGy* cm, and the mean effective equivalent dose was 5.08 mSv. DISCUSSION It is almost common knowledge that the duration of the contrast injection, or contrast column, should be approximately equal to the sum of the scan time and scan delay, as has previously been described(7). In addition, it has been demonstrated that a large iodine load is not warranted at a scan time less than 5 s, because that is much shorter than the duration of the contrast bolus(9). In the present study, the acquisition parameters applied yielded a mean scan time of 2.5 s, with a mean delay of 6.6 s and total scan times just above 9 s. That is quite well suited to an 11-s contrast column. Reducing the volume of contrast material employed has several advantages, and the current recommendation is to use the smallest volume and lowest dose of iodine that will yield a diagnostic result(11). Despite the controversy in the literature about the risk and incidence of contrast-induced kidney injury and its outcome(12,13), some authors have identified a relationship between kidney injury and the volume of contrast material used(14). In addition, from a financial point of view, reducing the volume of contrast material used translates to a reduction in health care system costs. A major point of discussion in the literature is which contrast material and iodine concentration achieve the best results. Initially, contrast material with a higher concentration of iodine was shown to produce better results(15). However, several authors(16-18) have reported that, at same iodine delivery rate (IDR)-defined as iodine concentration multiplied by injection rate(19)-there is no statistical difference among different iodine concentrations in terms of the contrast enhancement of the pulmonary arterial tree. In a recent study involving a porcine model, Behrendt et al.(20) showed that, at the same IDR, the greatest intravascular enhancement was achieved with moderate iodine concentrations. In a previous study, Behrendt et al.(19) had shown that, for chest imaging, better contrast enhancement was achieved with 300 mg I/mL than with 370 mg I/mL, at the same IDR. In the present study, we employed an IDR of 1.35 g I/s, with a total iodine dose of 15 g. In all patients, we achieved a arterial density superior than the minimum attenuations of blood required to see all acute and chronic pulmonary venous thromboemboli (93 and 211 HU, respectively), as calculated by Wittram(21). Because the major quality issue in CT angiography is intravascular enhancement and not tissue enhancement, the total dose of contrast is not an important factor. The major factor to consider is the acquisition time (scan time plus delay time) in relation to the contrast bolus. The studies mentioned above have shown that a major index to consider is the IDR, rather than the contrast iodine concentration or total iodine dose. In a study conducted by Wu et al.(10), a total iodine dose of 9.6 g was injected in patients receiving a low dose (30 mL) of contrast material, with no reduction in image quality, and the authors concluded that the volume of contrast can and should be significantly reduced in carefully selected groups of patients. In the present study, no patient selection would be made on any clinical basis. Therefore, we chose to use 50 mL of contrast at a 4.5 mL/s injection rate, given that, as previously discussed, this combination would prove to be the most suitable in terms of the technical aspects. In addition, with no prior clinical selection, we figured that some problems related to cardiovascular conditions, such as cardiac insufficiency, could cause a loss of image quality at lower contrast volumes. We did not encounter the inspiration artifact discussed in the literature(7,22), which could render a nondiagnostic result, in any of the cases evaluated here. In accordance with recent data(7,9,23,24), we used low kilovoltage acquisition in our patients, with only three exceptions in which, due to clinical conditions, we used a tube voltage of 120 kV in order to avoid nondiagnostic results. We maintained the mean radiation dose at the suggested levels(7). The major limitation of our study is the absence of a control group for the comparison of image quality, which limits the statistical analysis. Because the idea was to assess the image quality obtained with the proposed protocol alone, we did not work with a comparative control population. In fact, the main idea was to show that good image quality can be achieved at a lower contrast volume, taking into consideration only the technical aspects discussed earlier, without patient selection. Other limitations of our study are related to the small size of the patient population, as well as to its origin. Because ours is an outpatient clinic, we have not worked with patient populations that are clinically quite complicated. CONCLUSIONS It seems that PCTA can be performed in a 64-MDCT scanner with 50 mL of iodinated contrast material in unselected patient populations, regardless of patient characteristics, and still produce images of high quality. Further clinical studies, involving larger patient samples in outpatient, emergency, and hospital settings, are needed in order to validate this protocol. REFERENCES 1. Wittram C, Meehan MJ, Halpern EF, et al. Trends in thoracic radiology over a decade at a large academic medical center. J Thorac Imaging. 2004;19:164-70. 2. Remy-Jardin M, Remy J, Wattinne L, et al. Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single-breath-hold technique - comparison with pulmonary angiography. Radiology. 1992;185:381-7. 3. PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990;263:2753-9. 4. van Rossum AB, Pattynama PM, Ton ER, et al. Pulmonary embolism: validation of spiral CT angiography in 149 patients. Radiology. 1996;201:467-70. 5. Remy-Jardin M, Remy J, Deschildre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology. 1996;200:699-706. 6. Dalrymple NC, Prasad SR, El-Merhi FM, et al. Price of isotropy in multidetector CT. Radiographics. 2007;27:49-62. 7. Hartmann IJ, Wittenberg R, Schaefer-Prokop C. Imaging of acute pulmonary embolism using multi-detector CT angiography: an update on imaging technique and interpretation. Eur J Radiol. 2010;74:40-9. 8. Patel S, Kazerooni EA, Cascade PN. Pulmonary embolism: optimization of small pulmonary artery visualization at multi-detector row CT. Radiology. 2003;227:455-60. 9. Faggioni L, Neri E, Sbragia P, et al. 80-kV pulmonary CT angiography with 40 mL of iodinated contrast material in lean patients: comparison of vascular enhancement with iodixanol (320 mg I/mL) and iomeprol (400 mg I/mL). AJR Am J Roentgenol. 2012;199:1220-5. 10. Wu CC, Lee EW, Suh RD, et al. Pulmonary 64-MDCT angiography with 30 mL of IV contrast material: vascular enhancement and image quality. AJR Am J Roentgenol . 2012;199:1247-51. 11. Laville M, Juillard L. Contrast-induced acute kidney injury: how should at-risk patients be identified and managed? J Nephrol. 2010;23:387-98. 12. Bruce RJ, Djamali A, Shinki K, et al. Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol. 2009;192:711-8. 13. Li J, Solomon RJ. Creatinine increases after intravenous contrast administration: incidence and impact. Invest Radiol. 2010;45:471-6. 14. Trivedi H, Foley WD. Contrast-induced nephropathy after a second contrast exposure. Ren Fail. 2010;32:796-801. 15. Langenberger H, Friedrich K, Plank C, et al. MDCT angiography for detection of pulmonary emboli: comparison between equi-iodine doses of iomeprol 400 mgI/mL and iodixanol 320 mgI/mL. Eur J Radiol. 2009;70:579-88. 16. Behrendt FF, Plumhans C, Keil S, et al. Contrast enhancement in chest multidetector computed tomography: intraindividual comparison of 300 mg/ml versus 400 mg/ml iodinated contrast medium. Acad Radiol. 2009;16:144-9. 17. Keil S, Plumhans C, Behrendt FF, et al. MDCT angiography of the pulmonary arteries: intravascular contrast enhancement does not depend on iodine concentration when injecting equal amounts of iodine at standardized iodine delivery rates. Eur Radiol. 2008;18:1690-5. 18. Mühlenbruch G, Behrendt FF, Eddahabi MA, et al. Which iodine concentration in chest CT? - a prospective study in 300 patients. Eur Radiol. 2008;18:2826-32. 19. Behrendt FF, Mahnken AH, Stanzel S, et al. Intraindividual comparison of contrast media concentrations for combined abdominal and thoracic MDCT. AJR Am J Roentgenol. 2008;191:145-50. 20. Behrendt FF, Pietsch H, Jost G, et al. Identification of the iodine concentration that yields the highest intravascular enhancement in MDCT angiography. AJR Am J Roentgenol. 2013;200:1151-6. 21. Wittram C. How I do it: CT pulmonary angiography. AJR Am J Roentgenol. 2007;188:1255-61. 22. Gosselin MV, Rassner UA, Thieszen SL, et al. Contrast dynamics during CT pulmonary angiogram: analysis of an inspiration associated artifact. J Thorac Imaging. 2004;19:1-7. 23. Heyer CM, Mohr PS, Lemburg SP, et al. Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology. 2007;245:577-83. 24. Schueller-Weidekamm C, Schaefer-Prokop CM, Weber M, et al. CT angiography of pulmonary arteries to detect pulmonary embolism: improvement of vascular enhancement with low kilovoltage settings. Radiology . 2006;241:899-907. 1. MD, Radiologist at the Central de Diagnóstico Ribeirão Preto (Cedirp), Ribeirão Preto, SP, Brazil 2. Medical Physicist at the Central de Diagnóstico Ribeirão Preto (Cedirp), Ribeirão Preto, SP, Brazil Mailing Address: Dr. Henrique Simão Trad Cedirp - Central de Diagnóstico Ribeirão Preto Avenida Nove de Julho, 1656, Jardim América Ribeirão Preto, SP, Brazil, 14020-170 E-mail: hsimtrad@gmail.com Received November 3, 2014. Accepted after revision June 1, 2015. Study conducted at the Central de Diagnóstico Ribeirão Preto (Cedirp), Ribeirão Preto, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554