Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 48 nº 5 - Sep. / Oct. of 2015
Vol. 48 nº 5 - Sep. / Oct. of 2015
|
ICONOGRAPHIC ESSAY
|
|
Study of the skin anatomy with high-frequency (22 MHz) ultrasonography and histological correlation |
|
|
Autho(rs): Elisa de Oliveira Barcaui1; Antonio Carlos Pires Carvalho2; Juan Piñeiro-Maceira3; Carlos Baptista Barcaui4; Heleno Moraes5 |
|
|
Keywords: Ultrasonography; Dermatology; Skin; Histology. |
|
|
Abstract: INTRODUCTION
A series of recent studies published in Brazil have highlighted the relevance of ultrasonography in the diagnosis and treatment of several diseases(1-15). Ultrasonography is utilized in dermatology since the 1970's to evaluate skin thickening(16). The development of apparatuses with frequency > 15 MHz has allowed for the identification of the different layers and structures of the skin and adnexal, considerably widening the use of the method in cases of dermatological diseases. High-frequency apparatuses have low penetration and, consequently, excellent resolution for visualization of superficial structures(17). The skin presents its own characteristics according to the anatomical region, age and race. The knowledge of anatomy and morphology is fundamental for a perfect assessment of the structures observed at ultrasonography(18). The present essay is aimed at demonstrating the correlation between the sonographic study and histological analysis of the normal skin, facilitating the understanding and the diagnosis of dermatological diseases. THE SKIN The skin is composed of two layers, namely, dermis and epidermis. Because of the proximity and reactional behavior of the subcutaneous tissue in the different pathological processes, some authors consider it as a third layer(19). Approximately 95% of the epidermis is composed of cells called keratinocytes that synthesize a protein called keratin. Keratinocytes form four layers that undergo continuous transformation. From the bottom to the surface, such layers are the following: basal, spinous, granular and corneous layers. Melanocytes, Langerhans and Merkel cells form the remaining 5%(18,20). Fibroblasts, dermal dendritic cells, mastocytes and macrophages constitute the main cell components of the dermis. Extracellular components include collagen and elastic fibers, and amorphous fundamental substance. The dermis is divided into two compartments: papillary dermis and reticular dermis. The connective tissue is the most abundant component of this region (70%) and is constituted of collagen fibers. In the papillary dermis these fibers are more delicate and fine as compared with those of the reticular dermis where such fibers present like thicker bundles of fibers(19). Between the epidermis and the dermis there is the basal membrane zone, a macromolecules mesh that connects the basal layer keratinocytes with the collagen fibers of the papillary dermis(20). The subcutaneous tissue is composed of adipocytes presenting globose cytoplasm without vacuoles. The fat lobules are separated by fibrotic septa crossed by small vessels(21) (Figure 1A). 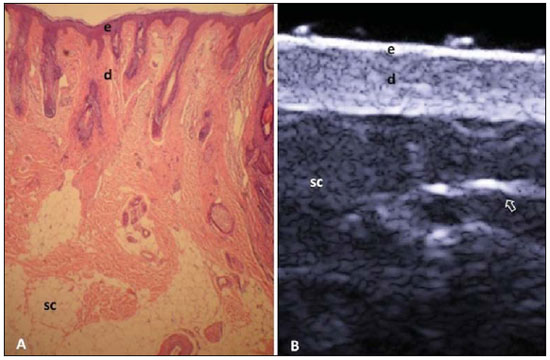 Figure 1. Non glabrous skin anatomy. A: Normal skin histology. B: High-frequency ultrasonography (HFUS), crosssectional view. Epidermis (e), dermis (d) and subcutaneous tissue (sc) with presence of fibrotic septa (arrow). The echogenicity of each layer depends on its main component: keratin (epidermis), collagen (dermis) and fat lobules (subcutaneous). At the sonographic image, the epidermis is seen as a hyperechoic line, the dermis as a less bright hyperechoic band and the subcutaneous layer as a hypoechoic layer with the presence of hyperechoic fibrotic septa inside(16) (Figure 1B). The echogenicity and the thickness of the dermis are variable, according to the patient's age (Figure 2). In neonates, it is slightly hypoechoic. In individuals of advanced age or intense actinic damage, a hypoechoic area called sub-epidermal low echogenic band is observed between the dermis and the epidermis, representing a probable sonographic manifestation of elastosis and edema in the papillary dermis (Figure 3). Some authors propose that the measurement of such a band could quantify the actinic damage(22). 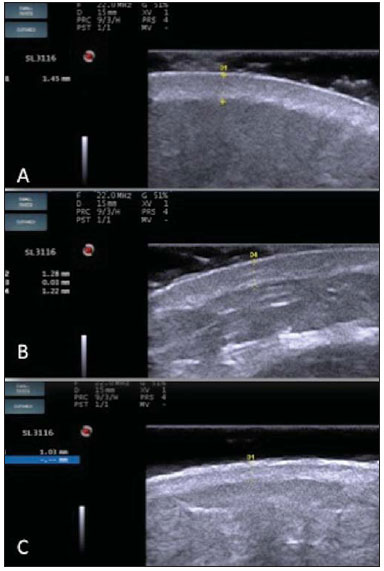 Figure 2. HFUS, cross sectional view, anterior region of the right forearm in consanguineous patients with a single skin phototype. A: 3-year-old patient. Thin epidermis and 1.45 mm-thick dermis. B: 25-year-old patient. Dermis measuring 1.22 mm in thickness. C: 55-year-old patient. Epidermal thickening and 1.03 mm-thick dermis. 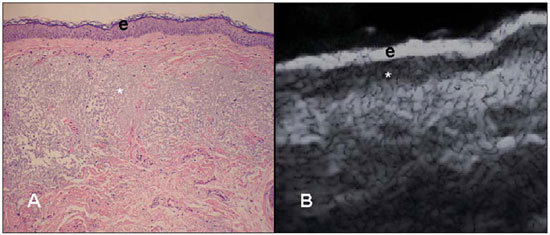 Figure 3. A: Histology. Epidermis (e), low-echogenicity subepidermal band corresponding to elastosis present in the connective tissue (asterisk). B: HFUS, crosssectional view. TOPOGRAPHIC ASSESSMENT Glabrous skin - palmo-plantar region Histologically, an additional epidermal layer is observed between the granular and corneous layers, called stratum lucidum. The cells of this layer are nucleated and called transitional cells(18) (Figure 4A). At high-frequency ultrasonography (HFUS), the epidermis of this anatomical area is seen as a bilaminar hyperechoic structure(23), possibly resulting from the contrast between the epidermis itself and a very thick and compact stratum corneum. The other skin layers are similar to the non glabrous skin (Figure 4B). 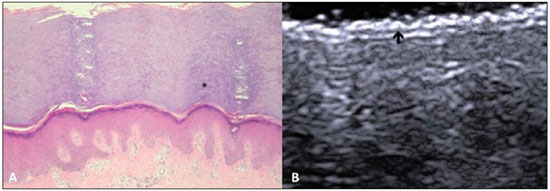 Figure 4. Glabrous skin. A: Histology. Thicker corneous layer and presence of stratum lucidum (asterisk). B: HFUS, cross-sectional view. Hyperechoic epidermis with bilaminar appearance (arrow). Scalp and hair shaft The hair follicle is a dynamic microstructure attached to the skin, responsible for the production of hair, in constant self-regeneration. For this reason, it presents a cyclic behavior. Periods of high mitotic activity and cell differentiation (anagenous phase) are interrupted by a remodelling phase (catagenous phase), followed by a quiescent period (telogen phase), with a subsequent growth restart (Figure 5A). 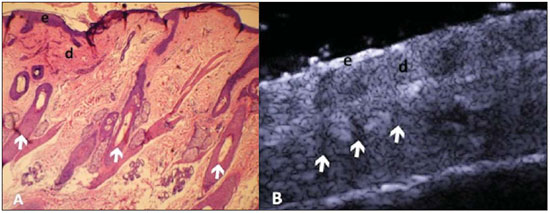 Figure 5. Scalp. A: Histology, longitudinal section. B: HFUS, longitudinal view. Epidermis (e), dermis (d), oblique hypoechoic bands corresponding to hair follicles (arrows). At HFUS, the appearance of the scalp skin layers is similar to the skin of other anatomical sites: hyperechoic line (epidermis), hyperechoic band (dermis) and hypoechoic band (subcutaneous). More deeply, a hypoechoic band corresponding to the galea is observed and, immediately after, the calvarium demonstrated by an intensely hyperechoic line. Longitudinal hypoechoic structures corresponding to the hair follicles are observed on the scalp images. Depending on the phase of the hair growth cycle, such structures are observed at different skin layers, as follows: in the telogen phase, the bulb is located in the dermis, while in the anagenous phase, the bulb is located in the subcutaneous tissue(24) (Figure 5B). The scalp skin of the frontal region is thinner than the occipital region, and the hair follicles density is variable(24) (Figure 6). 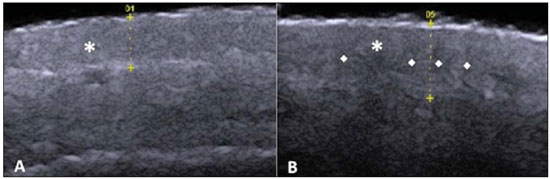 Figure 6. HFUS, longitudinal view, scalp. Male, 41-year-old patient. Variation of the skin thickness and in the number of hair follicles. A: Frontal region. B: Occipital region. Dermis (asterisks), hair follicle (diamonds). The anagenous phase of the hair follicle originates the terminal hair shaft composed of cuticle, cortex and medulla(20) (Figure 7A). HFUS, longitudinal view allows for differentiating a trilaminar hyperechoic structure, probably corresponding to the arrangement of keratin layers (Figure 7B). 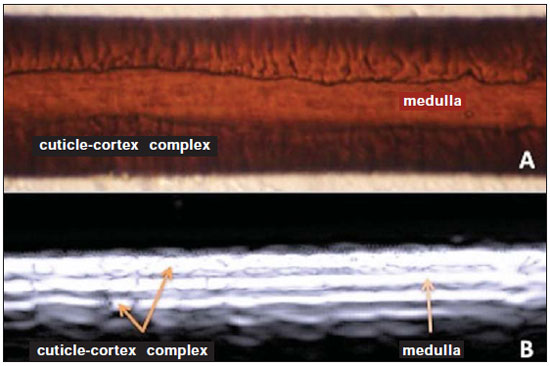 Figure 7. Hair shaft. A: Histology. Cortex-cuticle-medulla structure. B: Longitudinal view, trilaminar. Ungual unit The ungual unit has five components, as follows: matrix, ungual lamina, cuticle, ungual bed and ungual folds (proximal, lateral and distal) (19,20) (Figure 8A). 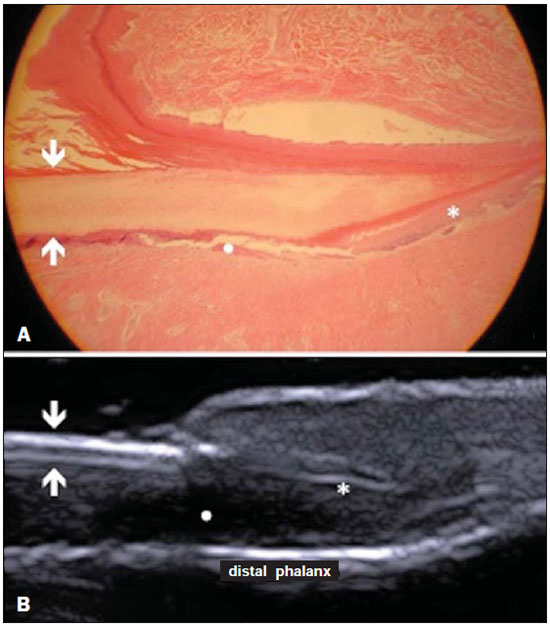 Figure 8. Normal ungual unit, longitudinal section. A: Histological section. B: HFUS. Ventral plate (downward arrow), dorsal plate (upward arrow), ungual matrix (asterisk), ungual bed (bold circle). At HFUS, the ungual lamina is subdivided into dorsal and ventral plates, generating a bilaminar, hyperechoic aspect separated by a thin hypoechoic line. A hypoechoic ungual bed is beneath the ungual lamina. The echogenic matrix may be observed at the proximal aspect of the ungual bed. A hypoechoic line corresponding to the bone of the distal phalanx is beneath the ungual bed(18,25) (Figure 8B). DISCUSSION The first step for the interpretation of the sonographic findings is the recognition of the different skin structures. In a single individual, it is possible to observe distinctive sonographic patterns, depending on the studied anatomical site. Apparatuses with frequencies > 15 MHz allow for the study of the skin and adnexa, since the skin layers and structures can be distinguished. However, apparatuses with frequencies > 20 MHz present better imaging resolution for the study of superficial structures. In the sonographic evaluation of the skin, one recommends the utilization of a thick gel layer between the transducer and the skin surface, in order to obtain a better focal point. A gelatinous cushion may be employed for the study of the ungual unit. It is important to utilize a delicate transducer, adaptable to the different contour of the body segments such as face and distal phalanx. The contact of the transducer with the skin must be the gentlest possible to avoid compression of the anatomical structures which, in this tissue, are thin and superficial. The hair on the region to be studied should be preferentially shaved and not cut with a scissor, in order to allow for a better contact between the transducer and the skin. Considering that different scalp diseases develop with alteration of the hair shaft, this can also be evaluated at HFUS. For the study of dermal lesions with crusts or marked keratinization it is recommended that such abnormalities are removed since they cause acoustic beam attenuation, reducing the accuracy of the examination. An appropriate skin evaluation utilizing HFUS includes defining the exact region to be studied, differentiate the skin layers, its thickness and vascularization, and identify possible associated pathological findings. CONCLUSION The arrival of HFUS has facilitated a more detailed study of the skin and adnexa, allowing for the diagnosis and definition of the treatment of dermatological diseases, which requires a deeper knowledge of the sonographic aspect of the normal skin. REFERENCES 1. Souza LRMF, De Nicola H, Yamasaki R, et al. Laryngeal schwannoma: a case report with emphasis on sonographic findings. Radiol Bras. 2014;47:191-3. 2. Resende DAQP, Souza LRMF, Monteiro IO, et al. Scrotal collections: pictorial essay correlating sonographic with magnetic resonance imaging findings. Radiol Bras. 2014;47:43-8. 3. Calixto AC, Brandão AHF, Toledo LL, et al. Prediction of preeclampsia by means of Doppler flowmetry of uterine artery and flow-mediated dilation of brachial artery. Radiol Bras. 2014;47:14-7. 4. Simão,MN. Carpal boss: a sonographic view. Radiol Bras. 2014;47(2):vii. 5. Terazaki CRT, Trippia CR, Trippia CH, et al. Synovial chondromatosis of the shoulder: imaging findings. Radiol Bras. 2014;47:38-42. 6. Arend CF. The carpal boss: a review of different sonographic findings. Radiol Bras. 2014;47:112-4. 7. Arend CF. Sonography of the iliotibial band: spectrum of findings. Radiol Bras. 2014;47:33-7. 8. Sakuno T, Tomita LM, Tomita CM, et al. Sonographic evaluation of visceral and subcutaneous fat in obese children. Radiol Bras. 2014;47:149-53. 9. Fernandes DA, Chagas ACP, Jesus AR, et al. Sonographic features associated with morbidity of chronic clinical presentations of schistosomiasis mansoni using the protocol proposed by the World Health Organization. Radiol Bras. 2013;46:1-6. 10. Martins FP, Vilela EG, Ferrari MLA, et al. Role of Doppler ultrasonography evaluation of superior mesenteric artery flow volume in the assessment of Crohn's disease activity. Radiol Bras. 2013;46:279-83. 11. Campos AG, Daneze ER, Terra Júnior JA, et al. Sonographic morphometry of the liver and biliary tract in porcine models submitted to experimental biliary obstruction. Radiol Bras. 2013;46:89-95. 12. Eifler RV. The role of ultrasonography in the measurement of subcutaneous and visceral fat and its correlation with hepatic steatosis. Radiol Bras. 2013;46:273-8. 13. Solha RS, Ajzen S, De Nicola H, et al. Morbidity of transrectal ultrasound guided prostate biopsy. Radiol Bras. 2013;46:71-4. 14. Araujo Júnior E, Simioni C, Nardozza LMM, et al. Prenatal diagnosis of Beckwith-Wiedemann syndrome by two- and three-dimensional ultrasonography. Radiol Bras. 2013;46:379-81. 15. Alves MPT, Fonseca COP, Granjeiro JM, et al. Carpal tunnel syndrome: comparative study between sonographic and surgical measurements of the median nerve in moderate and severe cases of disease. Radiol Bras. 2013;46:23-9. 16. Kleinerman R, Whang TB, Bard RL, et al. Ultrasound in dermatology: principles and applications. J Am Acad Dermatol. 2012;67:478-87. 17. Wortsman X, Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol. 2010;62:247-56. 18. Venus M, Waterman J, McNab I. Basic physiology of the skin. Surgery. 2010;28:469-72. 19. Elder DE, Elenitsas R, Johnson BL Jr, et al. Lever's histopathology of the skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. 20. Lai-Cheong JE, McGrath JA. Structure and function of skin, hair and nails. Medicine. 2009;37:223-6. 21. Wortsman X. Common applications of dermatologic sonography. J Ultrasound Med. 2012;31:97-111. 22. Gniadecka M. Effects of ageing on dermal echogenicity. Skin Res Technol. 2001;7:204-7. 23. Wortsman X. Ultrasound in dermatology: why, how, and when? Semin Ultrasound CT MR. 2013;34:177-95. 24. Wortsman X, Wortsman J, Matsuoka L, et al. Sonography in pathologies of scalp and hair. Br J Radiol. 2012;85:647-55. 25. Wortsman X, Jemec GB. Ultrasound imaging of nails. Dermatol Clin. 2006;24:323-8. 1. Master Fellow degree, Program of Post-graduation in Medicine (Radiology), Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil 2. Associate Professor, Department of Radiology, Coordinator for the Program of Post-graduation in Medicine (Radiology), Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil 3. Collaborating Professor of Dermatology and Pathological Anatomy, School of Medical Sciences, Universidade do Estado do Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil 4. Associate Professor of Dermatology, School of Medical Sciences, Universidade do Estado do Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil 5. Associate Professor of Pathological Anatomy, School of Medical Sciences, Universidade do Estado do Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil Mailing Address: Dra. Elisa de Oliveira Barcaui Rua Farme de Amoedo, 106, Ipanema Rio de Janeiro, RJ, Brazil, 22420-020 E-mail: ebarcaui@gmail.com Received April 6, 2014. Accepted after revision September 3, 2014. Study developed in the Department of Radiology at School of Medicine, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554