Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 48 nº 3 - May / June of 2015
Vol. 48 nº 3 - May / June of 2015
|
ORIGINAL ARTICLE
|
|
Prevalence of exclusive lower extremity metastases at 18F-NaF PET/CT |
|
|
Autho(rs): Monique Beraldo Ordones1; Agnes Araujo Valadares2; Paulo Schiavom Duarte3; Heitor Naoki Sado3; Marcos Santos Lima4; Giovanna Carvalho4; Marcelo Tatit Sapienza5; Carlos Alberto Buchpiguel6 |
|
|
Keywords: PET/CT; 18F-NaF; Scintigraphy; Bone; Metastasis. |
|
|
Abstract: INTRODUCTION
Bone scintigraphy is one of the most utilized nuclear medicine imaging methods, offering the advantages of whole-body assessment at a single scanning session, relatively low cost and high sensitivity(1-3). Currently, technetium-99m-methylene diphosphonate (99mTc-MDP) is the main radiopharmaceutical utilized in most imaging centers, but other radiopharmaceuticals may be utilized in the assessment of bone alterations. In this context, the increasing utilization of F-18 sodium fluoride is highlighted (18F-NaF)(2-4). 18F-NaF, a positron emitter whose half life is 110 minutes, was the first tracer utilized for skeleton images acquisition, introduced by Blau et al. in 1962 and approved for clinical utilization by the U.S. Food and Drug Administration in 1972(5,6). In the mid-1970s, fluoride was replaced by 99mTc-polyphosphonate, both due the wider availability of 99Mo/99mTc generators and the more appropriate characteristics of 99mTc-polyphosphonates for utilization in gamma chambers. The 18F-NaF uptake mechanism is similar to that of 99mTc-MDP, with 18F- ions exchange with OH- on the surface of the bone hydroxyapatite matrix, but with better pharmacokinetic characteristics, including rapid blood clearance and greater fluoride uptake by the bone (about two-fold greater than that of 99mTc-MDP), resulting in a better target to non-target ratio in a short time interval(7,8). The pharmacokinetic superiority of fluoride associated with a higher spatial resolution and higher sensitivity of PET/CT allows for the formation of better quality images as compared with planar scintigraphy and single-photon emission tomography. In Brazil, 18F-NaF is produced since 2008 by Instituto de Pesquisas Energéticas e Nucleares, and the scan modality has been performed at several Brazilian institutions. Thus, a more appropriate evaluation of the utilization of this technique is indispensable and undoubtedly there is a necessity for definition of the better imaging protocol, in order to reduce costs, allowing for a more rapid dissemination of the method in the country. In the literature, there is no protocol defining the body extent to be studied, so frequently whole-body images are acquired similarly to bone scintigraphy. On the other hand, the investigation of metastases by 18F-FDG PET/CT or with other radiopharmaceuticals is frequently performed only up to the distal portion of the lower limbs because of the low prevalence of exclusive lower extremity tumors in most cases. Due to the short half life of the radiopharmaceutical, the reduction of the aquisition time resulting from the smaller scan extent may imply an increase in the number of scans performed with a same radiopharmaceutical activity and, consequently, a reduction of involved costs. Objective To evaluate the prevalence of exclusive lower-extremity metastases, more specifically those below the femur, at 18F-NaF PET/CT. MATERIALS AND METHODS One thousand consecutive 18F-NaF PET/CT studies were retrospectively reviewed. The scans were performed in the period from June 2011 to January 2013. The images with exclusive uptake in lower limbs were initially classified into three categories, as follows: poorly suggestive of mestastasis; undefined; suggestive of metastasis. The presumptive diagnoses based on such exclusive peripheral uptakes were later established either by means of evaluation of other studies or by reanalysis of the PET/CT images by a radiologist with experience in musculoskeletal system images. On the basis of such presumptive diagnoses, the patients were divided into two categories, as follows: probably malignant uptake and probably benign uptake. On its turn, probably malignant uptakes were classified into femoral and below-the-femur. The PET/CT images were acquired in a Discovery GE 690 apparatus. Each patient received a 18F-NaF activity corresponding to 185 MBq (5 mCi) and the acquisitions occurred about 60 minutes after the radiopharmaceutical administration. The 3D time-of-flight whole-body PET images were acquired with one minute per bed position, slice thickness = 15 cm and 3 cm slices overlapping (corresponding to 13 to 15 bed positions, depending on the patient's height). Whole body CT was performed with 120 kVp, 30 mAs, 0.5 second/rotation, pitch = 1.0, and slice thickness = 3.75 mm. RESULTS Twenty-six patients presented exclusive lower extremity uptake. In two patients, such uptakes were initially classified as metastases, in 13, as undefined, and in 11, as poorly suggestive of metastasis. Later, one of the cases of exclusive uptakes classified as metastasis in the major trochanter of the right femur (Figure 1A) was classified as probably benign, because pelvic CT image acquired after 15 months demonstrated an area of possible heterotopic calcification in this region (Figure 1B); in the other case classified as metastasis, malignancy located in the right femoral neck was confirmed by magnetic resonance imaging (MRI) (Figure 2) - the patients presented with a lung neoplasm. Amongst the 13 patients with exclusive lower limb uptake classified as undefined, two cases were classified as probably malignant in the images reanalysis and in the evaluation of other studies. In one of such cases, the uptake was located in the left intertrochanteric region and was considered as suggestive of secondary compromise at MRI (Figure 3) - the patient presented with breast cancer with metastases in the liver and lung. In the other case, the uptake was located in the distal region of the right femur and the malignancy was confirmed by the PET/CT computed tomography image itself, characterizing bone infiltration by metastasis from melanoma in adjacent soft tissue (Figure 4) - the patient presented with multiple nodular areas in soft tissues compatible with involvement by the underlying disease. The other cases of undefined uptake were classified as probably benign by the reanalysis of PET/CT images or by further imaging studies. The 11 cases of uptake originally classified as poorly suggestive of metastasis were classified as benign as follow-up and images reanlysis. Thus, only two out 1,000 patients presented exclusive lower extremity bone uptake suggesting bone metastasis, both in the proximal third of the femora, and a third patient presented multiple metastases from melanoma in soft tissues, with one of such metastases infiltrating the distal region of the left femur. 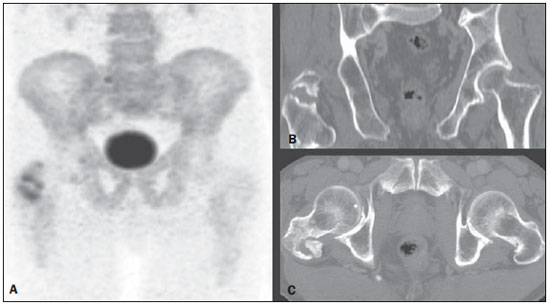 Figure 1. 18F-NaF PET image (A) shows osteogenic reaction in the region of the major trochanter and intertrochanteric sulcus of the right femur, suggesting secondary involvement by the underlying disease. Coronal (B) and axial (C) pelvic CT performed after 15 months revealing possible heterotopic calcification in this region, classified as a probably benign lesion. 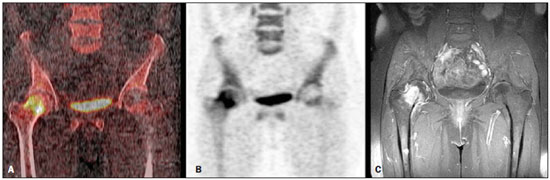 Figure 2. Coronal fusion PET/CT (A) and PET (B) with 18F-NaF show osteogenic reaction at right femoral head and neck, suspicious for bone involvement secondary to the underlying disease. Pelvic MRI coronal section T1-weighted image with fat suppression after intravenous gadolinium injection (C) shows expansile lesion infiltrating the bone marrow of the right femoral head and neck, suggestive of secondary finding. Also a medullary lesion is observed in the femoral diaphysis suspicious for secondary involvement. 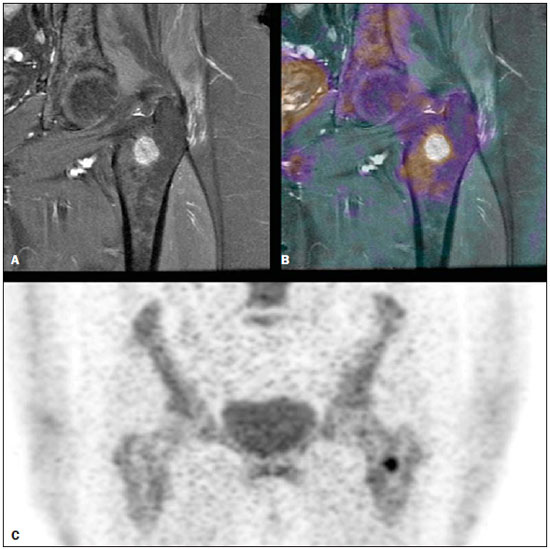 Figure 3. MRI coronal section of left hip – T1-weighted image with fat suppression after intravenous gadolinium injection (A) shows nodular lesion measuring 1.7 cm in the intertrochanteric region. 18F-NaF PET – coronal section (B) shows focal hyper-uptake in the same region, suspicious for secondary involvement; coronal 18F-NaF PET and MRI fusion of left hip (C) reveals correspondence of metabolic and anatomic findings. 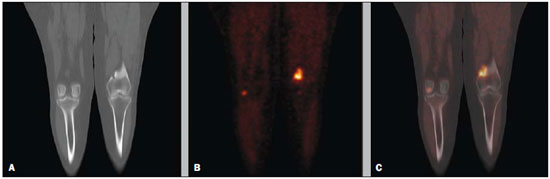 Figure 4. Coronal CT (A), PET (B) and 18F-NaF PET/CT fusion (C) images show osteogenic reaction in the distal metaphyseal region of the left femur, suggestive of metastatic bone infiltration originating from melanoma. Osteogenic reaction of probable osteodegenerative origin is also observed. DISCUSSION In spite of the fact that the presence of single metastases in the skeleton is not an uncommon finding, the prevalence of exclusive lower extremity metastasis detected at bone scintigraphy is low(9,10). Such a prevalence has already been evaluated at scans with 99mTc-MDP using a gamma chamber(9,10), and some case reports describe such finding, that is considered to be rare, principally in cases where the metastasis is located below the femora(11,12). However, the current protocols for 99mTc-MDP bone scintigraphy keep recommending whole-body imaging despite the low prevalence of metastasis in lower extremities(13,14). With the introduction of 18F-NaF bone scintigraphy performed in a PET/CT apparatus, one has raised the question about the extent of the body segment to be studied, considering that the acquisition of a smaller extent of the body might lead to reduction of the images acquisition time and, consequently, to a higher number of scans to be performed delivering a same radiopharmaceutical activity. In spite of the fact that the Society of Nuclear Medicine protocol on 18F-NaF PET/CT(8) does not specify the body extent to be assessed, the topic regarding patient's positioning mentions the protocol of that same society on the utilization of 18F-FDG PET/CT, recommending the scanning from the skull base to the root of the thighs(15). Additionally, some services(16) have recommended the scanning to be done from the skull base to the mid thigh, possibly on the basis of the 18F-FDG PET/TC protocols(15,17). Despite such a discussion, up to the present moment there is no study in the literature evaluating the prevalence of exclusive bone metastasis in lower extremities, particularly below the femora, detected at 18F-NaF PET/CT. In the present retrospective analysis of 1,000 consecutive scans performed in the authors' institution, only three cases of exclusive bone metastasis in lower extremities were observed, but in one of the patients, the metastasis was actually to soft tissue with extension to the adjacent bone tissue. Thus, in the present series the prevalence of exclusive bone metastasis in lower extremities was of only 0.2%, and the two described metastases occurred in the proximal third of the femora. Therefore, as demonstrated by previous studies about 99mTc-MDP bone scintigraphy(9,10) and, as confirmed the present results obtained with 18F-NaF PET/CT, the prevalence of exclusive bone metastasis in lower extremities, particularly below the femora, is low, thus the scanning up to the knees migh be appropriate in most cases. Such a reduction in the extent of the body to be assessed during scan may reduce the acquisition time in up to about 4 minutes (25%), which implies a higher number of scans to be performed with delivery of a same activity, given the short radiopharmaceutical half life (110 minutes). Another issue to be taken into consideration in the analysis of the present series is that, although the bone metastases in those two patients were exclusive in lower extremities, in at least one of them this was not the only metastatic site as the patient with breast cancer presented also with lung and liver metastases. Thus, in sucha a patient the area of bone metastasis is not considered, by definition(18), to be an exclusive metastasis and, therefore, its presence does not change the disease stage. So, as far as the disease stage definition is considered, the imaging of lower extremities could only change such parameter in 0.1% of the patients in the present study. CONCLUSION The prevalence of exclusive uptake in lower extremities suggesting metastasis is low, and exclusive bone metastases predominantly occur in the femora. Thus the 18F-NaF PET/CT scan on the body segment from the head to the knees is appropriate in most cases where such a scan is requested for investigation of metastases. REFERENCES 1. Brenner AI, Koshy J, Morey J, et al. The bone scan. Semin Nucl Med. 2012;42:11-26. 2. Beheshti M, Vali R, Waldenberger P, et al. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging. 2008;35:1766-74. 3. Iagaru A, Mittra E, Yaghoubi SS, et al. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy: results of the pilot-phase study. J Nucl Med. 2009;50:501-5. 4. Blau M, Nagler W, Bender MA. Fluorine-18: a new isotope for bone scanning. J Nucl Med. 1962;3:332-4. 5. Blau M, Ganatra R, Bender MA. 18 F-fluoride for bone imaging. Semin Nucl Med. 1972;2:31-7. 6. Grant FD, Fahey FH, Packard AB, et al. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68-78. 7. Segall G, Delbeke D, Stabin MG, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813-20. 8. Koizumi M, Yoshimoto M, Kasumi F, et al. Comparison between solitary and multiple skeletal metastatic lesions of breast cancer patients. Ann Oncol. 2003;14:1234-40. 9. Boxer DI, Todd CE, Coleman R, et al. Bone secondaries in breast cancer: the solitary metastasis. J Nucl Med. 1989;30:1318-20. 10. Duarte PS. Metatarsal metastasis from lung cancer read as a benign process on Tc-99m MDP scintigraphy. Clin Nucl Med. 2007;32:501-3. 11. Wu B, Xiu Y, Jiang L, et al. SPECT/CT imaging of patella metastasis from a squamous carcinoma of the lung. Clin Nucl Med. 2013;38:125-7. 12. Bombardieri E, Aktolun C, Baum RP, et al. Bone scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30:BP99-106. 13. Donohoe KJ, Henkin RE, Royal HD, et al. Procedure guideline for bone scintigraphy: 1.0. Society of Nuclear Medicine. J Nucl Med. 1996;37:1903-6. 14. Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885-95. 15. Even-Sapir E, Mishani E, Flusser G, et al. 18F-Fluoride positron emission tomography and positron emission tomography/computed tomography. Semin Nucl Med. 2007;37:462-9. 16. Boellaard R, O'Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181-200. 17. Hoshi M, Takada J, Ieguchi M, et al. Prognostic factors for patients with solitary bone metastasis. Int J Clin Oncol. 2013;18:164-9. 18. Langsteger W, Balogova S, Huchet V, et al. Fluorocholine (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: prospective comparison of diagnostic performance determined by masked reading. Q J Nucl Med Mol Imaging. 2011;55:448-57. 1. MD, Resident, Center of Nuclear Medicine, Instituto de Radiologia do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HCFMUSP), São Paulo, SP, Brazil 2. Nuclear Medicine Physician at Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil 3. PhDs, Physician Assistants, Service of Nuclear Medicine, Instituto do Câncer do Estado de São Paulo Octavio Frias de Oliveira (Icesp), São Paulo, SP, Brazil 4. Physician Assistants, Service of Nuclear Medicine, Instituto do Câncer do Estado de São Paulo Octavio Frias de Oliveira (Icesp), São Paulo, SP, Brazil 5. Private Docent, Professor, Department of Radiology and Oncology, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil 6. Private Docent, Full Professor, Department of Radiology and Oncology, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil Mailing Address: Dra. Monique Beraldo Ordones Instituto do Câncer do Estado de São Paulo - Setor de Medicina Nuclear Avenida Doutor Arnaldo, 251, Sumaré São Paulo, SP, Brazil, 01255-000 E-mail: moniqueordones@yahoo.com.br Received June 15, 2014. Accepted after revision November 10, 2014. Study developed at Service of Nuclear Medicine, Instituto do Câncer do Estado de São Paulo Octavio Frias de Oliveira (Icesp) and at the Center of Nuclear Medicine, Instituto de Radiologia do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554