Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 48 nº 2 - Mar. / Apr. of 2015
Vol. 48 nº 2 - Mar. / Apr. of 2015
|
ORIGINAL ARTICLE
|
|
Effects of iodinated contrast agent, xylocaine and gadolinium concentration on the signal emitted in magnetic resonance arthrography: a samples study |
|
|
Autho(rs): Yvana Lopes Pinheiro da Silva1; Rita Zanlorensi Visneck Costa2; Kátia Elisa Prus Pinho3; Ricardo Rabello Ferreira4; Sueliton Miyamoto Schuindt5 |
|
|
Keywords: Magnetic resonance arthrography; Contrast media; Magnetic resonance imaging; Xylocaine. |
|
|
Abstract: INTRODUCTION
The use of magnetic resonance imaging (MRI) has increased in the last years, both for the greater availability of apparatuses and its wide application in different clinical settings(1). This method is considered as the gold standard for acquisition of gadolinium-enhanced abdominal images(2), cardiac images for cardiac mass and volume quantification(3), and is also widely used in the evaluation of joints, allowing for direct visualization of relevant anatomical structures, including ligaments, menisci and periarticular soft tissues. Studies show that the delimitation of many intra-articular structures, the visualization of the normal articular anatomy, and the description of abnormalities are reinforced by the presence of articular effusion. At magnetic resonance arthrography (MRA), such an articular effusion is iatrogenically obtained, combining the advantages of the joint distension, the high-contrast resolution and the multiplanar acquisition capability of the method(4). Before the MR images acquisition, a needle is inserted into the joint to be studied and the intra-articular location is confirmed by means of either computed tomography (CT)- or fluoroscopically-guided injection of a small volume of iodinated contrast agent. Then, the diluted paramagnetic contrast/saline solution is injected into the intra-articular region and the images acquisition is performed. The technique might include a simultaneous injection of xylocaine without vasoconstrictor combined with the contrast agent in order to relieve the pain, and to help the patient to remain still during the scanning(5). According to the literature, the paramagnetic contrast dilution with iodinated contrast agent might have a significant effect on the quality of the MRA scan(6). Thus, the present study was aimed at investigating if the presence of iodinated contrast agent and xylocaine without vasoconstrictor in the solution injected into the joint affects the image quality and the signal emitted by the paramagnetic contrast agent, besides evaluating which is the most appropriate paramagnetic contrast concentration in MRA. MATERIALS AND METHODS An in vitro study was developed with three different gadodiamide (gadolinium) concentrations, as follows: 2.5 mmol/L, 5.0 mmol/L and 10.0 mmol/L, and each concentration was diluted in 100 mL saline solution (0.9% sodium chloride). Subsequently, nine 10 mL syringes received 8 mL of those solutions and were added as follows: syringes numbers 1, 2 and 3 received 1.5 mL saline solution; syringes 4, 5 and 6 received 1.5 mL non-ionic iodinated contrast agent; and syringes 7, 8 and 9 received 1.5 mL xylocaine without vasoconstrictor. Thus, the final volume in each syringe was 9.5 mL. Each syringe contents was transferred to standard polystyrene 15 mL flasks which were inserted into an expanded polystyrene block for immobilization during the images acquisition (Figure 1). Additionally, six control tubes were utilized - three out of them filled with 9.5 mL saline solution and the other three flasks filled with vegetal soya oil to aid in the samples orientation during the study (Figure 2). 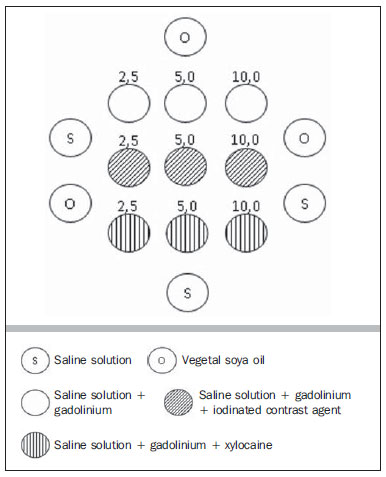 Figure 1. Representation of the test samples. The numbers represent the gadolinium concentration (in mmol/L) utilized in each of the flasks.  Figure 2. Test samples. Neither color plastic materials, glues with high proton concentration, nor other materials with magnetic susceptibility very different from the solutions were utilized in the making of the samples, since these types of materials could produce local inhomogeneity and susceptibility artifacts(7). Images acquisition The samples' images were acquired with a 1.5 T MRI apparatus utilizing a skull coil for acquisition of T1-weighted fast spin echo pulse sequences with fat saturation, TR = 416.7 ms, TE = 7.9 ms, matrix = 256 × 256 pixels, slice thickness = 3.0 mm and FOV = 21. The flasks were kept closed during the images acquisition in order to minimize possible changes in the solutions (for example, evaporation). The air conditioning temperature was set at 18°C, providing the same room temperature and, consequently the same temperature of the samples during the process of images acquisition. Images analysis The images were transferred and analyzed with the software Advantage Workstation GE 2010. The region of interest (ROI) was utilized to define the central region of the sample and to quantify the signal emitted by the different gadolinium dilutions and concentrations at the images, determining the average value of pixels in different areas of grey level in the ROI, i.e. the mean brightness of the ROI. The ROIs were identified in the center of the flasks where the signal intensities were measured and amplitude values were recorded. Three different sizes of ellipse-shaped ROI (6 mm, 9 mm and 12 mm in diameter), were utilized to calculate the mean signal intensity as measured on these three ROI sizes. The charts were designed in accordance with the amplitude values (signal intensity on the ROI) versus gadolinium concentration for each one of the three sample mixtures (saline solution, iodinated contrast agent and xylocaine). RESULTS With T1-weighted, fast spin eco sequence (TR/TE = 416.7/7.9) with fat saturation (Figure 3), the peak signal amplitude was produced at the gadodiamide 2.5 mmol/L concentration diluted in regular saline solution for all the ROI sizes. Gadodiamide dilution with iodinated contrast agent and xylocaine resulted in decreased signal amplitude for all the ROI sizes, as compared with gadodiamide dilution with saline solution. 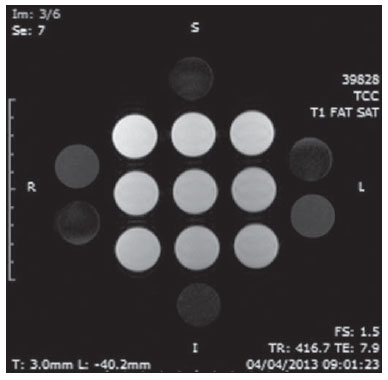 Figure 3. Image of the test object acquired with T1-weighted FSE pulse sequence (TR/TE = 416.7/7.9) with fat saturation. For ROI with 6 mm in diameter (Figure 4), the signal intensity with gadolinium diluted in iodinated contrast agent decreased 18.56% at 2.5 mmol/L concentration, 20.13% at 5.0 mmol/L concentration, and 23.21% at 10.0 mmol/L concentration, as compared with their respective concentrations diluted in saline solution only. For dilution with xylocaine, the decrease in the gadolinium signal intensity was of 27.80% at 2.5 mmol/L concentration, 29.21% at 5.0 mmol/ L concentration, and 27.68% at 10.0 mmol/L concentration, also compared with their respective concentrations diluted in saline solution only. 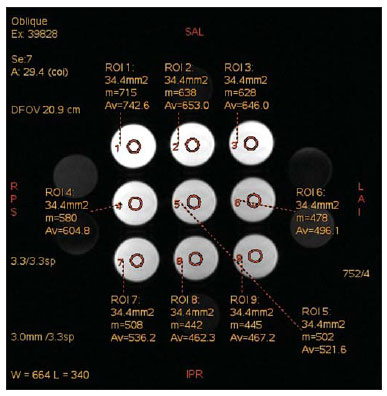 Figure 4. Image of the test object analyzed with a ROI with 6 mm in diameter. For ROI with 9 mm in diameter (Figure 5), the signal intensity with gadolinium diluted in iodinated contrast agent decreased 18.57% at 2.5 mmol/L concentration, 20.20% at 5.0 mmol/L concentration, and 23.48% at 10.0 mmol/L concentration, as compared with their respective concentrations diluted in saline solution only. For dilution with xylocaine, the decrease in the gadolinium signal intensity was of 27.97% at 2.5 mmol/L concentration, 29.37% at 5.0 mmol/L concentration, and 27.87% at 10.0 mmol/L concentration, also compared with their respective concentrations diluted in saline solution only. 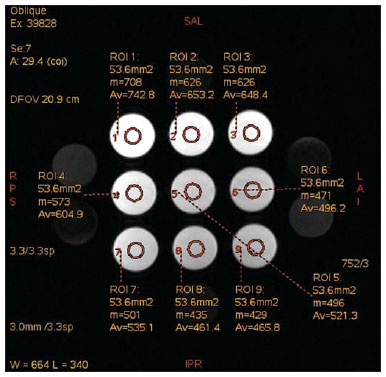 Figure 5. Image of the test object analyzed with a ROI with 9 mm in diameter. For ROI with 12 mm in diameter (Figure 6), the signal intensity with gadolinium diluted in iodinated contrast agent decreased 18.42% at 2.5 mmol/L concentration, 20.38% at 5.0 mmol/L concentration, and 23.01% at 10.0 mmol/L concentration, as compared with their respective concentrations diluted in saline solution only. For dilution with xylocaine, the decrease in the gadolinium signal intensity was of 27.72% at 2.5 mmol/L concentration, 29.20% at 5.0 mmol/L concentration, and 27.87% at 10.0 mmol/L concentration, also compared with their respective concentrations diluted in saline solution only. 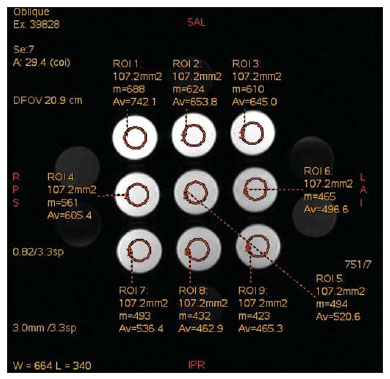 Figure 6. Image of the test object analyzed with a ROI with 12 mm in diameter. Figure 7 shows the decrease in gadodiamide signal intensity after dilution with iodinated contrast agent and xylocaine. One has considered that the signal emitted by these three gadodiamide solutions diluted in saline solution only represent the maximum intensities (100%) emitted by gadolinium for each of the different concentrations, because the saline solution is utilized only as a diluent for the solution to be injected into the joint. The signal intensity with dilution in iodinated contrast agent was 81.48% for gadodiamide 2.5 mmol/L concentration, 79.76% for 5.0 mmol/L concentration, and 76.76% for gadodiamide 10.0 mmol/L concentration. On the other hand, the signal intensity with dilution in xylocaine was 72.17% for gadodiamide 2.5 mmol/L concentration, 70.74% for 5.0 mmol/L concentration, and 72.09% for gadodiamide 10.0 mmol/L concentration. 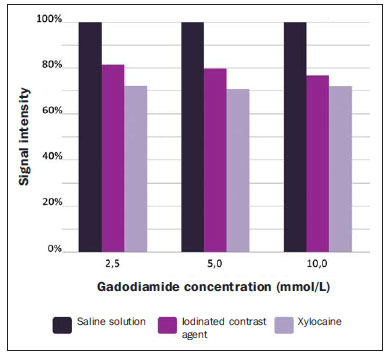 Figure 7. Comparison of the decrease in signal intensity considering the gadodiamide dilution in saline solution as maximum intensity. DISCUSSION Greater intrinsic soft tissue contrast resolution in association with non-exposure to ionizing radiation have made MRI an excellent choice for screening purposes(8), as well as in the evaluation of joints. MRA has been utilized for a detailed evaluation of internal joint derangements because of its capacity to describe small anatomical details, increasing the diagnostic accuracy. In many circumstances, MRA is superior to conventional non-contrast-enhanced MRI and to CT arthrography in the evaluation of several diseases affecting shoulders, knees, hip and other joints(9). Brown et al.(5) have developed an in vitro study, where three iodinated contrast agents were mixed and incubated with paramagnetic contrast agent. They observed that no gadolinium ion dissociated from the complex, even after addition of saline solution, xylocaine or epinephrine. Such results demonstrate that the mixture of contrast material, gadolinium and iodinated contrast agent is safe, and xylocaine and/or epinephrine might also be added for clinical purposes. According to Montgomery et al.(6), the literature reports inconsistency both in the gadolinium concentration utilized in MRA and in the amount of injected iodinated contrast agent and xylocaine. In the present study, the gadolinium concentrations were different (2.5 mmol/L, 5.0 mmol/L and 10.0 mmol/L) and the amounts of iodinated contrast agent (1.5 mL) and xylocaine (1.5 mL) remained equal, and are the same utilized by physicians who perform MRA in the diagnostic center where the present study was developed. The authors observed that the increased gadolinium concentration in the samples causes decrease in the gadolinium signal intensity for all the dilutions. According to Bushong(10), this occurs because if the gadolinium concentration in a determined area becomes extremely high, it is possible that the T2-weighting effect predominates even on a T2-weighted image, causing signal intensity loss on both image types. According to Montgomery et al.(6), this may be harmful, particularly in scans performed in joints with small volume of synovial fluid, as in the region of the wrist, since in such joints there is less contrast dilution. Therefore, the utilization of a gadolinium 2.5 mmol/L concentration allows for some dilution in the synovial fluid, which may even increase the signal intensity, according to the results reported by Montgomery et al.(6). The improvement in the gadolinium concentration and in the amount of iodinated contrast agent and xylocaine results in visually perceptible differences which may significantly affect the MRA diagnostic quality. Another implication of such data is that previous reports evaluating the MRA efficacy may have not utilized appropriate gadolinium concentrations and/or minimized the use of iodinated contrast agent and xylocaine. As a result, the MRA diagnostic relevance in relation to the other imaging modalities might have been underestimated(6). The present study demonstrates that the addition of iodinated contrast agent or xylocaine leads to a decrease in the signal emitted by the gadolinium, as suggested by Kopka et al.(11), who say that the iodinated contrast agent reduces the gadolinium T1 effect, although the exact mechanism of this action is still unknown. As regards xylocaine, up to this moment there is no comparative study demonstrating its effect on MRA images, probably because xylocaine is not as frequently utilized as iodinated contrast agent in this type of procedure. CONCLUSION The present study results demonstrate that the peak signal intensity was obtained with a gadodiamide concentration of about 2.5 mmol/L diluted in regular saline solution (Figures 4, 5 and 6). Thus, on the basis of the results and considering the presence of iodinated contrast agent and xylocaine without vasoconstrictor in the solution injected into the joint, a gadolinium concentration = 2.5 mmol/L is recommended. Gadolinium dilution in iodinated contrast agent and xylocaine has led to a reduction of respectively 20.76% and 28.34% in the signal intensity as compared with the samples with equal concentrations diluted in saline solution only. Such percentage values correspond to the calculated means for the three different gadolinium concentrations, diluted in iodinated contrast agent and xylocaine and to the different ROI sizes. Therefore, according to the present results, minimizing the use of iodinated contrast agent and xylocaine and/or the utilization of a gadolinium 2.5 mmol/L concentration diluted in saline solution will improve the sensitivity and specificity of MRA in the evaluation of internal joint derangements. REFERENCES 1. Galvão BVT, Torres LR, Cardia PP, et al. Prevalência de cistos simples e hemangiomas hepáticos em pacientes cirróticos e não cirróticos submetidos a exames de ressonância magnética. Radiol Bras. 2013;46:203-8. 2. Kim YH, Shin SS, Burke LMB, et al. Hemangioma hepático subcapsular com realce perilesional: achados de RM. Radiol Bras. 2010;43:384-8. 3. Barranhas AD, Santos AASMD, Coelho-Filho OR, et al. Cardiac magnetic resonance imaging in clinical practice. Radiol Bras. 2014;47:1-8. 4. Hajek PC, Sartoris DJ, Neumann CH, et al. Potential contrast agents for MR arthrography: in vitro evaluation and practical observations. AJR Am J Roentgenol. 1987;149:97-104. 5. Brown RR, Clarke DW, Daffner RH. Is a mixture of gadolinium and iodinated contrast material safe during MR arthrography? AJR Am J Roentgenol. 2000;175:1087-90. 6. Montgomery DD, Morrison WB, Schweitzer ME, et al. Effects of iodinated contrast and field strength on gadolinium enhancement: implications for direct MR arthrography. J Magn Reson Imaging. 2002;15:334-43. 7. Price RR, Axel L, Morgan T, et al. Quality assurance methods and phantoms for magnetic resonance imaging: report of AAPM Nuclear Magnetic Resonance Task Group No. 1. Med Phys. 1990;17:287-95. 8. Hernandes MA, Semelka RC, Elias Jr, et al. Whole-body MRI: comprehensive evaluation on a 48-channel 3T MRI system in less than 40 minutes. Preliminary results. Radiol Bras. 2012;45:319-25. 9. Choi JY, Kang HS, Hong SH, et al. Optimization of the contrast mixture ratio for simultaneous direct MR and CT arthrography: an in vitro study. Korean J Radiol. 2008;9:520-5. 10. Bushong SC. Magnetic resonance imaging: physical and biological principles. 3rd ed. Philadelphia, PA: Mosby; 2003. 11. Kopka L, Funke M, Fischer U, et al. MR arthrography of the shoulder with gadopentetate dimeglumine: influence of concentration, iodinated contrast material, and time on signal intensity. AJR Am J Roentgenol. 1994;163:621-3. 1. Radiology Technologist, Universidade Tecnológica Federal do Paraná (UTFPR), Curitiba, PR, Brazil 2. PhD in Sciences, Professor at Universidade Tecnológica Federal do Paraná (UTFPR), Curitiba, PR, Brazil 3. Fellow PhD degree in Sciences, Professor at Universidade Tecnológica Federal do Paraná (UTFPR), Curitiba, PR, Brazil 4. MD, Radiology and Ultrasonography Specialist at Centro Diagnóstico Água Verde (Cedav), Curitiba, PR, Brazil 5. Radiology Technologist, Graduate Student of Medicine, Universidade Federal do Paraná (UFPR), Curitiba, PR, Brazil Mailing Address: Yvana Lopes Pinheiro da Silva Rua Pedro Collere, 699, Vila Izabel Curitiba, PR, 80320-320, Brazil E-mail: yvanaa@gmail.com Received October 3, 2013. Accepted after revision September 3, 2014. Study developed at Centro Diagnóstico Água Verde (Cedav), Curitiba, PR, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554