Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 5 - Sep. / Oct. of 2014
Vol. 47 nº 5 - Sep. / Oct. of 2014
|
CASE REPORT
|
|
Aortic lesion simulating pulmonary disease: a case report |
|
|
Autho(rs): Ricardo Holderbaum do Amaral1; Vinícius Valério Silveira de Souza1; Carlos Schuler Nin1; Cesar Adrian Alvarez Pedraza1; Júlia Biegelmeyer2; Antonio Carlos Maciel3 |
|
|
Keywords: Aorta; Aortic arch; Computed tomography. |
|
|
Abstract: INTRODUCTION
Penetrating aortic ulcer (PAU) was first described in 1934(1), and only in 1986 was characterized as a clinical and pathological entity(2), which, in association with its relative rarity, explains the wide unawareness of this condition. It is caused by ulceration of an atheromatous plaque, penetrating through the elastic lamina and progressing to the media, in association with aortic wall hematoma. It is part of acute aortic syndrome (AAS), together with aortic dissection, intramural hematoma and traumatic aortic rupture, which may present similar clinical manifestations, hence the high relevance of a rapid and accurate radiological diagnosis(2,3). CASE REPORT A 76-year-old woman attended the emergency unit of a university hospital complaining of sudden onset of dyspnea in association with right costal margin pain for one week. The patient presented a significant clinical history with cardiac failure secondary to hypertension, emphysematous chronic obstructive pulmonary disease (COPD), and heavy smoking. At physical examination, the patient presented subtle perioral cyanosis, diffuse expiratory wheezes and crackles in the lower third of the chest, bilaterally, with no other significant alteration. The cardiac enzymes curve was normal and electrocardiography indicated left ventricle hypertrophy, with no other abnormality. Frontal chest radiography performed with the patient in dorsal decubitus demonstrated the presence of an opacity on the projection of the left upper lobe, adjacent to the aortic arch that was found elongated and sinuous, with enlargement of the cardiac area, diffuse and bilateral interstitial infiltrates, and presence of residual calcified micronodules (Figure 1). 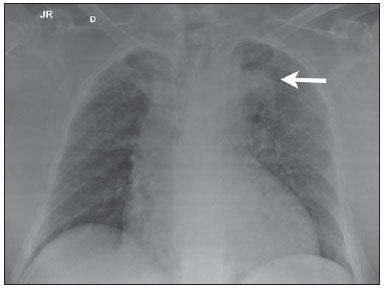 Figure 1. Frontal chest radiographic image demonstrating opacity in the left upper lobe, adjacent to the aortic arch (arrow). Non-contrast-enhanced and contrast-enhanced computed tomography revealed the presence of calcified atheromatous plaques and focal dilatation with 2.6 cm in diameter on the posterolateral aspect of the aortic arch, filled by contrast agent, in communication with the aortic lumen, associated with subintimal hematoma, compatible with PAU (Figures 2, 3 and 4). 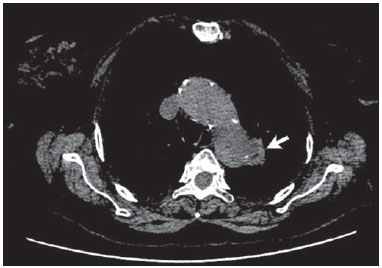 Figure 2. Contrast-enhanced computed tomography, pre-contrast phase showing saccular dilatation with 2.6 cm in diameter on the posterolateral aspect of the aortic arch, in association with subintimal hematoma (arrow). Axial section. 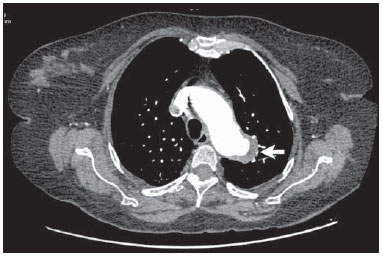 Figure 3. Contrast-enhanced computed tomography, arterial phase showing saccular dilatation with 2.6 cm in diameter on the posterolateral aspect of the aortic arch (arrow) filled by contrast agent and in communication with the aortic lumen, in association with subintimal hematoma. Axial section. 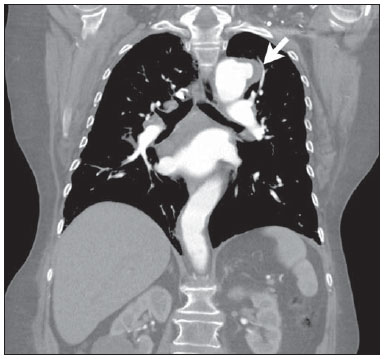 Figure 4. Contrast-enhanced computed tomography, arterial phase showing saccular dilatation with 2.6 cm in diameter on the posterolateral aspect of the aortic arch (arrow), filled by contrast agent and in communication with the aortic lumen, in association with subintimal hematoma. Coronal section. Because of the clinical condition and of the prohibitive surgical risk, option was made for clinical management in agreement with the patient and her family. A maximum systolic pressure of 120 mmHg was established as a goal, with management of comorbidities with emphasis on COPD stabilization. Until the present moment, 70 days after the first consultation, the patient is asymptomatic from the cardiovascular point of view, with no sign of lesion progression. DISCUSSION As a considerably less frequent condition than dissection (70%) and intramural hematoma (20%), PAU corresponds to 5% of AAS. Usually, this disease involves the descending aorta and rarely the aortic arch (0.1% of AAS)(3-6). PAU affects patients with advanced atherosclerosis, particularly the elderly and hypertensive individuals. Initially, the lesion is asymptomatic - an atheromatous ulceration restricted to the intimal layer. Later, the ulcer deepens, penetrating through the elastic lamina and progressing to the media with different degrees of hematoma. The degree of distension and weakening of the aortic wall caused by the hematoma may lead to the development of a saccular aneurysm and rupture(3,4). Contrast-enhanced computed tomography is the diagnostic method of choice in most cases. Findings include focal lesion with subintimal hematoma, located under an intimal layer that is often calcified and internally displaced. Frequently there is thickening or contrast uptake by the aortic wall, giving the appearance of an aggressive lesion(3). Despite the lower sensitivity for detecting small lesions, this method is satisfactory, considering that it can diagnose eventual extraluminal diseases, and computed tomography angiography acts as a complementary method in the evaluation of mural abnormalities(7). Transesophageal echocardiography has been successfully used and is highly sensitive and specific in the differential diagnosis of aortic disease(7). Rarely, multiple ulcers may be found(3). Scarce data are available about the natural history of this disease. However, some authors suggest a poorer prognosis than that for aortic dissection(2,4), with series reporting rupture in up to 40% of the cases(3). Its management still remains controversial. Surgical intervention is recommended in the presence of intramural hematoma expansion, signs of imminent rupture and hemodynamic instability(3). The presence of pain seems to be the most relevant clinical variable in such an evaluation(2). Currently, the management by means of endovascular prosthesis is preferred considering its less invasiveness as compared with the open repair(8). Endovascular prosthesis can be used even in cases of aortic rupture, with lower morbidity and mortality(4). However, in many cases the endovascular treatment of the ascending aorta and aortic arch is not feasible because of technical difficulties. In such cases, surgical procedure is the method of choice(2). Immediate intervention is not always required considering that, many times, the course of the disease is benign(9). The authors describe the case of a lesion that simulated a pulmonary disease at conventional radiography. Further investigation with computed tomography was fundamental for the diagnosis and for guiding the appropriate clinical approach. REFERENCES 1. Shennan T. Dissecting aneurysms. Medical Research Council Special Report Series No. 193. London: His Majesty's Stationary Office; 1934. 2. Stanson AW, Kazmier FJ, Hollier LH, et al. Penetrating atherosclerotic ulcers of the thoracic aorta: natural history and clinicopathologic correlations. Ann Vasc Surg. 1986;1:15-23. 3. Hayashi H, Matsuoka Y, Sakamoto I, et al. Penetrating atherosclerotic ulcer of the aorta: imaging features and disease concept. Radiographics. 2000;20:995-1005. 4. Piffaretti G, Tozzi M, Lomazzi C, et al. Endovascular repair of abdominal infrarenal penetrating aortic ulcers: a prospective observational study. Int J Surg. 2007;5:172-5. 5. Girela A, Barbosa F, Quiroga J. Treatment of penetrating aortic ulcer involving the aortic arch associated with lesion of the left main coronary artery. Rev Arg Cardiol. 2012;80:405-13. 6. Wells CM, Subramaniam K. Acute aortic syndrome. In: Subramaniam K, Park KW, Subramaniam B, editors. Anesthesia and perioperative care for aortic surgery. New York: Springer; 2011. p. 17-36. 7. Sommer T, Fehske W, Holzknecht N, et al. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996;199:347-52. 8. Novero ER, Metzger PB, Obregon J, et al. Tratamento endovascular das doenças da aorta torácica: análise dos resultados de um centro. Radiol Bras. 2012;45:251-8. 9. Harris JA, Bis KG, Glover JL, et al. Penetrating atherosclerotic ulcers of the aorta. J Vasc Surg. 1994;19:90-9. 1. MDs, Residents, Department of Radiology - Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, RS, Brazil 2. MD, Resident, Department of Internal Medicine - Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, RS, Brazil 3. PhD, Head of the Department of Radiology - Santa Casa de Misericórdia de Porto Alegre, MD, Radiologist, Unit of Radiology - Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil Mailing Address: Dr. Ricardo Holderbaum do Amaral Santa Casa de Misericórdia de Porto Alegre - Serviço de Radiologia Rua Professor Annes Dias, 295, Centro Histórico Porto Alegre, RS, Brazil, 90020-090 E-mail: rh.doamaral@gmail.com Received June 14, 2013. Accepted after revision October 25, 2013. Study developed at the Department of Radiology - Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, RS, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554