Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 5 - Sep. / Oct. of 2014
Vol. 47 nº 5 - Sep. / Oct. of 2014
|
REVIEW ARTICLE
|
|
Hepatobiliary contrast agents: differential diagnosis of focal hepatic lesions, pitfalls and other indications |
|
|
Autho(rs): Flávia Angélica Ferreira Francisco1; Antonio Luis Eiras de Araújo2; Jaime Araújo Oliveira Neto3; Daniella Braz Parente4 |
|
|
Keywords: Focal hepatic lesions; Hepatobiliary contrast; Magnetic resonance imaging. |
|
|
Abstract: INTRODUCTION
The characterization of focal liver lesions has a great clinical relevance. Magnetic resonance imaging (MRI) with intravenous contrast injection (extracellular gadolinium-based contras media commonly utilized in the radiological practice) is considered the best imaging method in the evaluation of such lesions. However, MRI does not allow for the diagnosis in all cases whose etiology remains undetermined. The utilization of hepatobiliary contrast agents increases the MRI accuracy, reducing the necessity of invasive diagnostic procedures intended to clarify the diagnosis of nonspecific lesions(1,2). The currently available hepatocyte-selective contrast media are the following: gadobenate dimeglumine (Gd-BOPTA - MultiHance®; Bracco, Milan, Italy) and gadoxetic acid (Gd-EOB-DTPA - Primovist®; Bayer-Schering, Berlin, Germany)(1,2). Such contrast agents are absorbed by hepatocytes via OATP1 transporter (polypeptide adenosintriphosphate-dependent organic anion transporter), the same as the bilirubin transporter. A fraction of hepatobiliary contrast agent is excreted by cMOAT into the biliary canaliculi (multispecific canalicular organic anion transporter)(1). Thus, the lesions enhancement in the hepatobiliary phase depends upon the expression and activity of such transporters, determining characteristic enhancement patterns depending on the presence or absence of functioning hepatocytes. In the hepatobiliary phase, the healthy liver is evenly enhanced, becoming hyperintense; the contrast agent uptake by the biliary tract occurs progressively, and the blood vessels become hyperintense as compared with the liver parenchyma as the contrast medium is no longer in the vascular compartment. Contrast uptake is also observed in focal liver lesions with functioning hepatocytes(1). Additionally, hepatobiliary contrast agents allow for evaluating the biliary tract(1-3). The usual dynamic study with arterial, portal and delayed phases is also performed with such contrast agents. So, hepatobiliary contrast agents combine the pharmacodynamic features of extracellular gadolinium (usual dynamic study) with the delayed hepatobiliary phase, adding functional information to the MRI study and enhancing its diagnostic accuracy(1,2,4-6). The pharmacokinetics and doses of gadobenate dimeglumine and gadoxetic acid are different. The gadobenate dimeglumine absorption by hepatocytes is of 3% to 5%, while the absorption of gadoxetic acid is of 50%. Consequently, the hepatobiliary phase acquisition time is different for each type of contrast agent and should be obtained 120 minutes after gadobenate dimeglumine administration (ranging between 1 and 3 hours), and 20 minutes after gadoxetic acid administration (ranging between 10 and 120 minutes)(1). The doses recommended for intravenous injection of such contrast agents are also different, corresponding to 0.1 mmol/kg (0.2 ml/kg) for gadobenate dimeglumine, and 0.025 mmol/kg (0.1 ml/kg) for gadoxetic acid(3). As the gadoxetic acid dose corresponds to one quarter of the habitual extracellular gadolinium dose, the arterial phase acquisition time is critical, requiring temporal precision methods, such as real time visualization of the contrast progression through the arterial system, for the success in this phase acquisition. On the other hand, the enhancement in the hepatobiliary phase is prolonged, allowing for acquisition of images with better spatial resolution, as well as its repetition in case of imaging artifacts(4). As gadobenate dimeglumine is utilized, it is recommended that the MRI study be performed as usual, including the dynamic study up to the delayed phase; then the procedure be interrupted and the patient returns after 120 minutes for acquisition of the hepatobiliary phase. As gadoxetic acid is utilized, the hepatobiliary phase occurs in 20 minutes, so it is recommended that the order of sequences acquisition be changed in order to optimize the acquisition time. Initially, the T1-weigthed sequences (in-phase, out-ofphase and with fat saturation) are performed. As necessary, heavily T2-weighted cholangiographic images should also be acquired before the contrast injection, since hepatobiliary contrast agents are excreted by the biliary tract and can shorten the T2-relaxation time. Subsequently, gadoxetic acid is intravenously injected and the dynamic study (arterial, portal and delayed phases) is performed. Diffusion- and T2-weighted sequences may be acquired after hepatobiliary contrast agent injection, considering that there is no significant interference effect. Finally, the hepatobiliary phase is acquired 20 minutes after gadoxetic acid administration(1,4). The use of hepatobiliary contrast agents requires some care. Focal liver lesions enhancement may be less intense during the dynamic study, particularly in the arterial phase, because the recommended dose of gadoxetic acid is lower than the habitual extracellular gadolinium dose(5). Additionally, patients with advanced cirrhosis may present less hepatobiliary contrast uptake as a result from liver dysfunction. Patients with hyperbilirubinemia may also present less hepatobiliary contrast uptake due to the direct competition between bilirubin and hepatobiliary contrast agents for a single transporter in the hepatocytes, which represents a limiting factor in patients with total bilirubin levels > 3 mg/dl(1,7,8). The flip angle also requires attention and must be around 25° to 40° in the acquisition of the hepatobiliary phase with the objective of enhancing the hepatic contrast(7). Gadobenate dimeglumine has biliary excretion around 3% to 5%, and renal excretion around 93 to 97%, and gadoxetic acid biliary and renal excretion on a 50/50 basis(8-10). Patients with advanced liver and kidney diseases alternatively compensate the contrast agents clearance by renal or biliary excretion, respectively. Patients with cirrhosis Child A or B do not present any significant alteration in the total clearance of hepatobiliary contrast agents; but in cirrhosis Child C, there is a decreased total clearance and increased half life, with compensatory increase of renal excretion(8). Adverse effects of hepatobiliary contrast agents rarely occur and, if present, are similar to the ones reported in the use of extra-cellular gadolinium. The risk for systemic nephrogenic fibrosis in the use of hepatobiliary contrast agents is rated as intermediate and must be avoided in cases of creatinine clearance < 30 ml/min(8). Main indications for hepatobiliary contrast include differentiation between focal nodular hyperplasia (FNH) and adenoma, characterization of hepatocelular carcinomas (HCCs), detection of small liver metastasis, assessment of biliary anatomy, and characterization of postoperative biliary fistulas. The imaging characterization of benign and malignant liver lesions is very important. Benign liver lesions are frequently found, even in patients with known neoplasia. The most frequent differential diagnoses for hypervascular lesions in patients with no hepatopathy include hemangioma, FNH and adenoma. Hemangiomas generally present typical imaging findings and are easily diagnosed by computed tomography or MRI with extracellular gadolinium contrast agent. However, the differentiation between FNH and adenoma is not always easy at conventional MRI, because both conditions may appear as nonspecific hypervascular lesions, generating anguish for the patient and challenging the physician, in addition to the cost and patient's anxiety with repeated examinations. The central scar of FNH is absent in 20% of cases, particularly in cases of small lesions. Signs of intratumoral hemorrhage and fat are not found in 30% to 40% of adenomas(11). It is extremely import to differentiate FNH from adenoma, especially in cases of lesions > 4.0 cm, considering that prognoses and approaches are different. FNH is a benign lesion that does not require any intervention, while adenoma presents risk for malignization, necrosis and bleeding which might require emergency surgery. Adenomas > 4 cm and those with symptoms related to intratumoral hemorrhage constitute surgical indication(12,13). Hepatobiliary contrast allow for the differentiation between FNH and adenoma in most cases, even in those of small lesions. FOCAL NODULAR HYPERPLASIA The typical FNH presents with septa and lobulated or microlobulated borders, with intermediate signal intensity on T1- and T2-weighted sequences, low lesion-organ contrast and homogeneous arterial contrast uptake, with decay in the subsequent phases, becoming isointense to the adjacent liver parenchyma. The presence of central scar markedly hyperintense on T2-weighted and hypointense on T1-weighted sequences, with no contrast uptake in the arterial phase and late contrast uptake is typical. However, in some cases, especially those of small lesions (without central scar), one cannot differentiate between FNH and adenoma due to overlapping imaging findings(1,4). FNH presents greater density of functioning hepatocytes than a healthy liver parenchyma, in association with abnormal bile ducts which do not communicate with greater bile ducts, with consequential slower biliary excretion as compared with the surrounding liver. Therefore, FNH presents contrast uptake greater or equal to the adjacent liver parenchyma in the hepatobiliary phase(4) (Figures 1 and 2). The central scar is generally hypointense in the hepatobiliary phase in 47% of cases, but a subtle contrast enhancement may be observed in some cases(1,9). 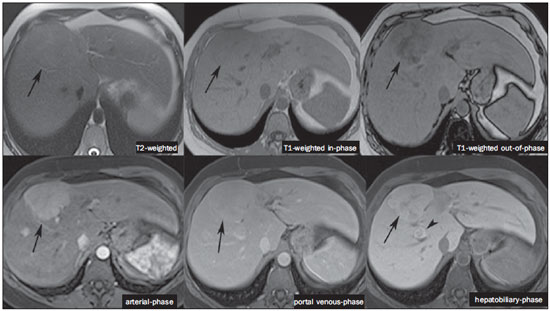 Figure 1. Female, 40-year-old patients presenting with liver steatosis and multiple, well-defined focal hypervascular lesions, with intermediate signal intensity on T2-weighted sequence, with poor lesion-organ contrast-enhancement. However, the presence of intralesional fat was detected on out-of-phase T1-weighted sequence. The presence of intralesional fat is not usually found in FNH and suggests the diagnosis of adenoma - adenomatosis, in the present case -, with a very different prognosis and approach. On the other hand, the lesions showed homogeneous hepatobiliary contrast uptake, hence the highest likelihood of the diagnosis of multiple FNHs. 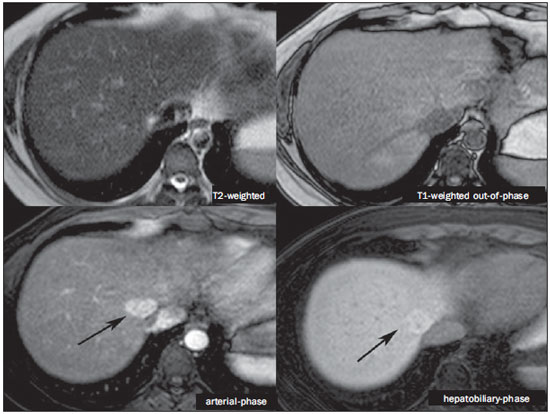 Figure 2. Female, 36-year-old, asymptomatic patient presenting with a hypervascular liver nodule to be clarified, without intralesional fat and without central scar. Homogeneous hepatobiliary contrast uptake indicates the diagnosis of FNH. ADENOMA Adenomas are well defined, homogeneous or heterogeneous lesions. The largest ones tend to present signal heterogeneity, with mild to moderate hypersignal on T2-weighted, hyposignal on T1-weighted sequences, homogeneous or heterogeneous arterial contrast-enhancement, late washout, and possible development of capsule(13). Adenomas are composed of hepatocytes containing glycogen and lipids surrounded by a capsule. Although containing functioning hepatocytes, there is a lack of biliary ducts resulting in deficiency in bilirubin and hepatobiliary contrast excretion. Additionally, adenomas present smaller expression of membrane transporters such as OATP1(1,2). Thus, in the hepatobiliary phase, most adenomas are hypointense in relation to the surrounding parenchyma (Figure 3). Rarely, there is hepatobiliary contrast uptake by adenomas and, in cases where it occurs, such an uptake tends to be preferentially peripheral in the hepatobiliary phase(1,2,4). 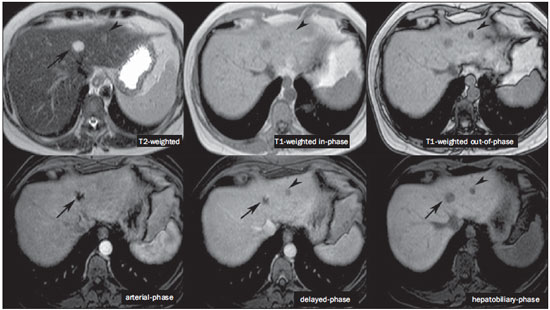 Figure 3. Female, 43-year-old patient undergoing follow-up for metastatic gastrointestinal stromal tumor, with liver nodules to be clarified. The smallest lesion (arrowheads) presents subtle hypersignal on T2-weighted and marked signal loss on out-of-phase T1-weighted sequence caused by the presence of intralesional fat. No hepatobiliary contrast uptake is observed. The presence of intralesional fat and the absence of hepatobiliary contrast uptake indicate a probable diagnosis of adenoma. The largest lesion (arrows) presents high signal intensity on T2-weighted, hyposignal on t1-weighted sequence, and nodular, peripheral and discontinuous uptake in the arterial-phase, and no hepatobiliary contrast uptake that is a typical hemangioma behavior. Hemangiomas do not contain functioning hepatocytes so uptake of this contrast medium is not observed. Also, in the delayed-phase, the fill-in pattern is not observed, which might occur with the utilization of hepatobiliary contrast agent. HEMANGIOMA Hemangiomas normally have a typical presentation at MRI with extracellular contrast and are not an indication for investigation with hepatobiliary contrast. At conventional MRI, hemangiomas present marked hypersignal on T2-weighted, hyposignal on T1-weighted sequences, discontinuous, nodular, peripheral contrast enhancement in the arterial phase, tending to centripetal fill-in by the contrast agent in the subsequent phases(13,14). However, considering that hemangiomas are common lesions, they will be frequently present on images acquired with hepatobiliary contrast for several reasons. Hemangiomas present the same imaging findings at dynamic studies with hepatobiliary contrast; however, in the delayed phase, as the hepatobiliary contrast medium is leaving the interstitium and entering into the functioning hepatocytes, the hemangioma fill-in might or might not occur in this phase, differing from its usual behavior with the use of extracellular gadolinium(14). Hemangiomas are formed by a clump of blood vessels and do not contain hepatocytes, therefore they do not present contrast enhancement during the hepatobiliary phase and appear hypointense in this phase(1,2,9,15) (Figure 4). A potential confusion factor is the fact that some hemangiomas may present subtle central contrast uptake during the early hepatobiliary phase because of the tendency to persistent centripetal enhancement at dynamic study, like in those with extracellular gadolinium(1). 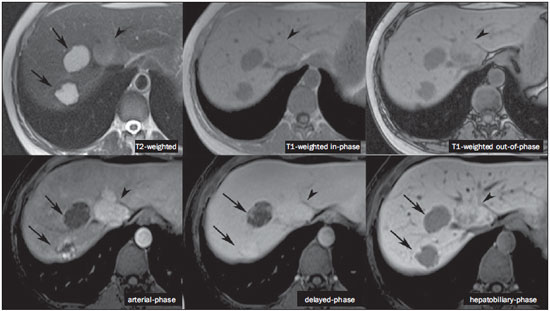 Figure 4. Female, 50-year-old patient with liver nodules to be clarified. The caudate lobe lesion (arrowheads) presents subtle hypersignal on T2-weighted sequence and signal loss on T1-weighted out-of-phase sequence caused by the presence of intralesional fat. Such a lesion shows intense and homogeneous contrast uptake in the arterial-phase, with decay in the portal and delayed phases, presenting greater hepatobiliary contrast uptake than the adjacent parenchyma, suggesting FNH as the first diagnostic hypothesis. Considering that the presence of intralesional fat in NFH is rare, the patient will be maintained under imaging follow-up. The lesions in segments VII and VIII (arrows) are similar, with marked hypersignal on T2-weighted, hyposignal on T1-weighted sequence, and nodular, peripheral and discontinuous uptake in the arterial phase, a characteristic of hemangiomas. HEPATOCELLULAR CARCINOMA Hepatobiliary contrast is also useful to increase the sensitivity and specificity in the detection of HCCs of all sizes, including those < 1 cm and those between 1 cm and 2 cm, in cirrhotic patients. The MRI sensitivity with the use of extracellular gadolinium to detect HCC ranges from 70% to 100%, but is much lower in cases of smaller HCCs(10,16). The characterization of lesions < 1 cm and those between 1 and 2 cm still represents a challenge, particularly concerning the differentiation between high-grade dysplastic nodules and early HCC(4). In cirrhosis, the hepatobiliary contrast uptake by the nodules depends on their differentiation stage and on the presence of functioning hepatocytes. Low-grade regenerative and dysplastic nodules present preferentially portal vascularization, contain functioning hepatocytes and, like the surrounding parenchyma, show hepatobiliary contrast uptake. High-degree dysplastic nodules lose the portal vascularization and start gaining abnormal arterial vascularization. Thus, high-grade dysplastic nodules tend to be hypovascular in the arterial and portal phases, but may also become hypervascular in the arterial phase in cases where the abnormal arterial vascularization is more developed. High-grade dysplastic nodules contain functioning hepatocytes and also demonstrate hepatobiliary contrast uptake in the same way as the surrounding parenchyma (Figure 5). Hepatobiliary contrast uptake by HCC also depends on its differentiation stage. Well-differentiated HCCs contain functioning hepatocytes and might show hepatobiliary contrast uptake. On the other hand, poorly-differentiated or undifferentiated hepatocarcinomas do not contain functioning hepatocytes and do not show hepatobiliary contrast uptake, remaining hypointense in relation to the surrounding parenchyma(2,10,17-19) (Figure 6). Hypointense lesions < 1 cm identified only in the hepatobiliary phase should be closely followed-up(10). 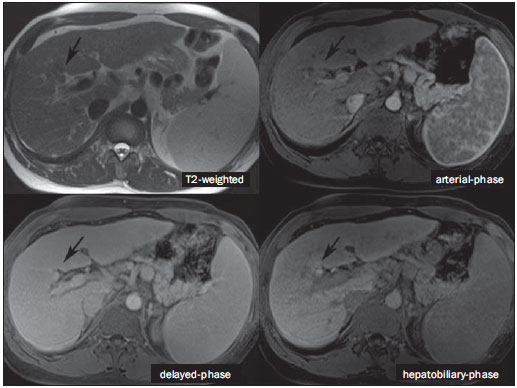 Figure 5. Male, 46-year-old patients presenting with chronic hepatopathy and liver nodule to be clarified, adjacent to the gallbladder, as seen at ultrasonography. Small nodules are observed adjacent to the gallbladder, with hyposignal on T2-weighted sequence, without expression on the other sequences and on the conventional dynamic study, but with hepatobiliary contrast uptake, leading to the diagnosis of regenerative nodules. Well-differentiated HCCs show hepatobiliary contrast uptake, requiring imaging follow-up. 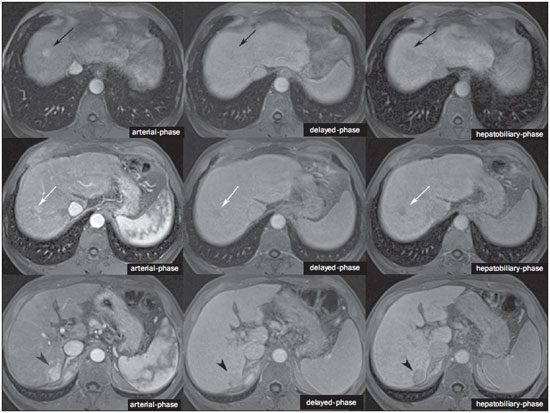 Figure 6. Male, 61-year-old patient presenting with chronic C virus hepatopathy. Two liver nodules are seen in the segment VIII (arrows) as well as a larger nodule, in the segment VI (arrowheads), all of them contrast-enhanced in the arterial-phase, washout in the delayed-phase, and without uptake in the hepatobiliary-phase, characterizing HCCs. Poorly differentiated or undifferentiated HCCs do not contain functioning hepatocytes so hepatobiliary contrast uptake is not observed. The different enhancement patterns depend on the histological grade of the HCCs and may be explained by the membrane transporters expression. HCCs with contrast enhancement equal to or more intense than that of the remainder liver parenchyma present high levels of OATP1 and cMOAT as compared with HCCs with hypoenhancement(20). Hepatobiliary contrast uptake by HCCs depends on the tumor differentiation stage and on the amount of functioning hepatocytes(2,4). The diagnostic performance of MRI in the detection of HCCs of all sizes increases with the utilization of hepatobiliary contrast agents(1,10). However, in cases of advanced cirrhosis, the contrast uptake by the liver parenchyma may be compromised by decreased hepatocytes function, which would result in reduction of the method's accuracy to detect HCCs(4,21). The differentiation between HCC and perfusion alterations may also represent a diagnostic challenge. Perfusional alterations present a signal similar to the one of the remainder hepatic tissue during the portal and hepatobiliary phases, while most HCCs, except the well-differentiated ones, present hyposignal in the hepatobiliary phase(22). The hepatobiliary phase may also be useful in the post-chemoembolization or post-radiofrequency ablation follow-up, considering that inflammatory reactions show hepatobiliary contrast uptake and residual HCC tends to not present contrast uptake(23). METASTASIS Hepatobiliary contrast increases the method's sensitivity to detect liver metastasis, particularly the small-sized ones. Metastases do not contain functioning hepatocytes or biliary ducts, and do not show contrast uptake during the hepatobiliary phase. As a result, the healthy hepatic tissue remains hyperintense and the metastasis, hypointense, which facilitates its detection(1,2). The utilization of such contrast agents increases the index of detection of hypo- and hypervascular metastases (Figure 7). Additionally, hepatobiliary contrast agents contribute to the diagnosis of small, benign focal lesions frequently found in patients with neoplasias, particularly FNH (Figure 8). Like in cirrhosis, perfusional alterations in patients with metastasis show contrast uptake in the hepatobiliary phase, differently from metastases(1). The use of hepatobiliary contrast agents in the staging of patients with colorectal neoplasia changes the clinical approach in up to 14% of patients with metastasis(2). 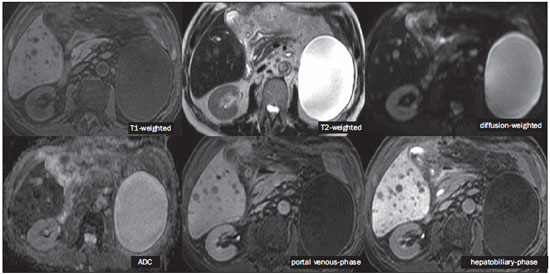 Figure 7. Male, 70-year-old patient presenting with colon cancer and multiple metastases, with hyposignal on T1-weighted, and subtle hypersignal on T2-weighted sequence. Hypovascular metastases with diffusion restriction. In the hepatobiliary-phase, the liver parenchyma shows contrast uptake and becomes hyperintense. The metastatic implants that do not contain hepatocytes become hypointense. Note the capacity of hepatobiliary contrast to detect very small lesions which cannot be visualized on the other sequences. 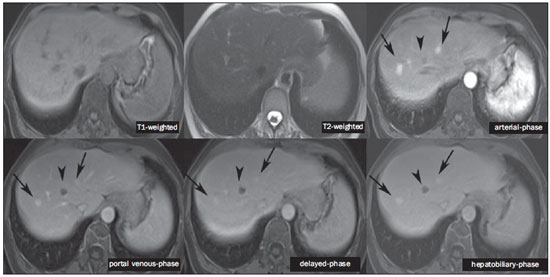 Figure 8. Female, 53-year-old patient presenting with colon cancer. Two hypervascular lesions (arrows) are seen with intermediate signal intensity on T1- and T2-weighted sequences, showing contrast uptake in the hepatobiliary-phase. Such lesions present functioning hepatocytes, suggesting FNHs as the main diagnostic hypothesis and ruling out the possibility of metastatic implants. The avascular lesion (arrowhead) is secondary to post-treatment alteration. ASSESSMENT OF THE BILIARY TRACT The imaging evaluation of the biliary system has been approached by a series of publications in the Brazilian radiological literature(24-31). The biliary excretion of hepatobiliary contrast agents allows for the anatomical and functional characterization of intra- and extrahepatic biliary tract. Such contrast agents shortens the T1 relaxation time of the bile and allows for the performance of a high-resolution T1-weighted cholangiography(4). The previous knowledge of the biliary anatomy and its variations becomes increasingly important in the preoperative planning, considering the complexity of the hepatic anatomy as well as of the more refined surgical techniques, which reduces the occurrence of postoperative complications(4). Also, hepatobiliary contrast-enhanced cholangiography allows for the accurate detection of postoperative complications such as biliary fistulas and bilomas which present progressive fill-in during the hepatobiliary phase. In the postoperative follow-up, inadvertent ductal ligation can also be easily recognized in the hepatobiliary phase as an abrupt interruption of the biliary tract(4,5). Other applications of hepatobiliary contrast agents include the evaluation of the biliary flow dynamics, the study of partial or complete biliary duct obstructions, and the localization of the stenosis site. The hepatobiliary contrast may contribute to the diagnosis of cholecystitis as the gallbladder is not filled by the contrast medium, differently from its habitual behavior with other contrast agents. The diagnosis of sphincter of Oddi dysfunction can be based on the finding of absent or delayed passage of the hepatobiliary contrast thru the ampulla of Vater. Hepatobiliary contrast allows for the differentiation between biliary lesions and extrabiliary cysts, since it delineates the biliary tract, demonstrating the communication of biliary cystic lesions with the bile ducts, and extrabiliary cystic lesions that do not communicate with bile ducts, such as pseudocysts, duodenal diverticula and duodenal duplication cysts(5). ASSESSMENT OF HEPATIC FIBROSIS Several studies are evaluating the relation between the degree of hepatic fibrosis in patients with cirrhosis As well as the hepatobiliary contrast enhancement with the objective of reducing the necessity of biopsies (currently considered a gold standard). Hepatocytes are responsible for the uptake and excretion of the hepatobiliary contrast medium, so their integrity is essential for the enhancement of the parenchyma in the hepatobiliary phase. In cirrhosis, hepatocytes are progressively replaced by fibrotic tissue, so that the more advanced the fibrosis, the smaller the hepatic parenchyma enhancement in the hepatobiliary phase. Additionally, as compared with healthy livers, cirrhotic livers present later enhancement peak and slower washout(32-37). Further potential hepatobiliary contrast applications include the evaluation of the functional hepatic reserve before partial hepatectomy; evaluation of live donor's hepatic function as well as evaluation of early liver failure after transplant(4). CONCLUSION In summary, hepatobiliary contrast increases the MRI accuracy and reduces the number of cases of undefined liver lesions. Imaging findings in the hepatobiliary findings should be always analyzed in the clinical context, considering the lesion signal characteristics on anatomical sequences. Main indications include: differentiation between FNH and adenoma; characterization of HCC in cirrhotic patients; detection of small liver metastases; evaluation of the biliary anatomy; and characterization of postoperative biliary fistulas. The utilization of hepatobiliary contrast agents may reduce the necessity of invasive diagnostic procedures as well as of further investigation with other imaging methods, and imaging follow-up, reducing costs and the anxiety of both patients and medical team. Further potential hepatobiliary contrast applications include evaluation of the functional hepatic reserve before partial hepatectomy; evaluation of live donor's hepatic function as well as evaluation of early liver failure after transplant. REFERENCES 1. Goodwin MD, Dobson JE, Sirlin CB, et al. Diagnostic challenges and pitfalls in MR imaging with hepatocyte-specific contrast agents. Radiographics. 2011;31:1547-68. 2. Ba-Ssalamah A, Uffmann M, Saini S, et al. Clinical value of MRI liver-specific contrast agents: a tailored examination for a confident non-invasive diagnosis of focal liver lesions. Eur Radiol. 2009;19:342-57. 3. Hammerstingl R, Zangos S, Schwarz W, et al. Contrast-enhanced MRI of focal liver tumors using a hepatobiliary MR contrast agent: detection and differential diagnosis using Gd-EOB-DTPA-enhanced versus Gd-DTPA-enhanced MRI in the same patient. Acad Radiol. 2002;9 Suppl 1:S119-20. 4. Seale MK, Catalano OA, Saini S, et al. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725-48. 5. Lee NK, Kim S, Lee JW, et al. Biliary MR imaging with Gd-EOBDTPA and its clinical applications. Radiographics. 2009;29:1707-24. 6. Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol. 2004;14:559-78. 7. Kim S, Mussi TC, Lee LJ, et al. Effect of flip angle for optimization of image quality of gadoxetate disodium-enhanced biliary imaging at 1.5 T. AJR Am J Roentgenol. 2013;200:90-6. 8. Gschwend S, Ebert W, Schultze-Mosgau M, et al. Pharmacokinetics and imaging properties of Gd-EOB-DTPA in patients with hepatic and renal impairment. Invest Radiol. 2011;46:556-66. 9. de Souza DA, Parente DB, de Araújo AL, et al. Modern imaging evaluation of the liver: emerging MR imaging techniques and indications. Magn Reson Imaging Clin N Am. 2013;21:337-63. 10. Parente DB, Perez RM, Eiras-Araújo A, et al MR imaging of hypervascular lesions in the cirrhotic liver: a diagnostic dilemma. Radiographics. 2012;32:767-87. 11. Bieze M, van den Esschert JW, Nio CY, et al. Diagnostic accuracy of MRI in differentiating hepatocellular adenoma from focal nodular hyperplasia: prospective study of the additional value of gadoxetate disodium. AJR Am J Roentgenol. 2012;199:26-34. 12. Bioulac-Sage P, Laumonier H, Sa Cunha A, et al. Hepatocellular adenomas. Liver Int. 2009;29:142. 13. Tiferes DA, D'Ippolito G. Liver neoplasms: imaging characterization. Radiol Bras. 2008;41:119-27. 14. Doo KW, Lee CH, Choi JW, et al. "Pseudo washout" sign in highflow hepatic hemangioma on gadoxetic acid contrast-enhanced MRI mimicking hypervascular tumor. AJR Am J Roentgenol. 2009;193:490-6. 15. Tamada T, Ito K, Yamamoto A, et al. Hepatic hemangiomas: evaluation of enhancement patterns at dynamic MRI with gadoxetate disodium. AJR Am J Roentgenol. 2011;196:824-30. 16. Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513-23. 17. Frericks BB, Loddenkemper C, Huppertz A, et al. Qualitative and quantitative evaluation of hepatocellular carcinoma and cirrhotic liver enhancement using Gd-EOB-DTPA. AJR Am J Roentgenol. 2009;193:1053-60. 18. Hanna RF, Aguirre DA, Kased N, et al. Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics. 2008;28:747-69. 19. Willatt JM, Hussain HK, Adusumilli S, et al. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247:311-30. 20. Kitao A, Zen Y, Matsui O, et al. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR imaging correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817-26. 21. Galvão BVT, Torres LR, Cardia PP, et al. Prevalence of simple liver cysts and hemangiomas in cirrhotic and non-cirrhotic patients submitted to magnetic resonance imaging. Radiol Bras. 2013;46:203-8. 22. Burke LMB, Vachiranubhap B, Tannaphai P, et al. Contrast enhancement of liver lesions in cirrhotic patients: a single institution crossover comparative study of two MR contrast agents. Preliminary results. Radiol Bras. 2011;44:147-50. 23. Motosugi U, Ichikawa T, Sou H, et al. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology. 2010;256:151-8. 24. Souza LRMF, Rodrigues FB, Tostes LV, et al. Imaging evaluation of congenital cystic lesions of the biliary tract. Radiol Bras. 2012;45:113-7. 25. Gössling PAM, Alves GRT, Silva RVA, et al. Spontaneous biloma: a case report and literature review. Radiol Bras. 2012;45:59-60. 26. Guimarães Filho A, Carneiro Neto LA, Palheta MS, et al. Caroli's disease complicated with liver abscess: case report. Radiol Bras. 2012;45:362-4. 27. Queiroz HMC, Costa FA, Campos Junior MM, et al. Arterial embolization in the treatment of hemobilia after hepatic trauma: a case report. Radiol Bras. 2012;45:63-4. 28. Campos AG, Daneze ER, Terra Júnior JA, et al. Sonographic morphometry of the liver and biliary tract in porcine models submitted to experimental biliary obstruction. Radiol Bras. 2013;46:89-95. 29. Rocha MS. Liver abscesses secondary to acute cholangitis. Radiol Bras 2013;46(2):xi. 30. Hollanda ES, Torres US, Gual F, et al. Spontaneous perforation of gallbladder with intrahepatic biloma formation: sonographic signs and correlation with computed tomography. Radiol Bras. 2013;46:320-2. 31. Maia MCA, Amaro AP, Oliveira EC, et al. Portal cholangiopathy: case report. Radiol Bras. 2014;47:51-3. 32. Norén B, Forsgren MF, Dahlqvist Leinhard O, et al. Separation of advanced from mild hepatic fibrosis by quantification of the hepatobiliary uptake of Gd-EOB-DTPA. Eur Radiol. 2013;23:174-81. 33. Tsuda N, Okada M, Murakami T. Potential of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) for differential diagnosis of nonalcoholic steatohepatitis and fatty liver in rats using magnetic resonance imaging. Invest Radiol. 2007;42:242-7. 34. Dahlqvisq Leinhard O, Dahlström N, Kihlberg J, et al. Quantifying differences in hepatic uptake of the liver specific contrast agents Gd-EOB-DTPA and Gd-BOPTA: a pilot study. Eur Radiol. 2012;22:642-53. 35. Lee WJ, Cha SH, Kim MY, et al. Quantitative evaluation of the hepatic parenchymal change in patients with chronic liver disease using Gd-EOB-DTPA-enhanced MRI: comparison with normal liver. J Korean Soc Radiol. 2011;64:49-55. 36. Watanabe H, Kanematsu M, Goshima S, et al. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusionweighted MR imaging - preliminary observations. Radiology. 2011;259:142-50. 37. Tsuda N, Okada M, Murakami T. New proposal for the staging of nonalcoholic steatohepatitis: evaluation of liver fibrosis on Gd-EOB-DTPA-enhanced MRI. Eur J Radiol. 2010;73:137-42. 1. MD, Resident of Radiology and Imaging Diagnosis, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil 2. Physician at Unit of Radiology and Imaging Diagnosis - Rede D'Or, Instituto D'Or de Pesquisa e Ensino (IDOR) and Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil 3. Physician at Unit of Radiology and Imaging Diagnosis - Rede D'Or and Instituto D'Or de Pesquisa e Ensino (IDOR), Rio de Janeiro, RJ, Brazil 4. PhD, Physician at Unit of Radiology and Imaging Diagnosis - Rede D'Or, Instituto D'Or de Pesquisa e Ensino (IDOR) and Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil Mailing Address: Dra. Flávia Angélica Ferreira Francisco Rua Cristóvão Xavier Lopes, 131, ap. 306, Coelho Neto Rio de Janeiro, RJ, Brazil, 21530-120 E-mail: flaviangel@gmail.com Received July 23, 2013. Accepted after revision October 22, 2013. Study developed at Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554