Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 4 - July / Aug. of 2014
Vol. 47 nº 4 - July / Aug. of 2014
|
CASE REPORT
|
|
Encapsulating peritonitis: computed tomography and surgical correlation |
|
|
Autho(rs): Juliana Santos Kadow1; Carla Jeronimo Peres Fingerhut1; Vinicius de Barros Fernandes1; Klaus Rizk Stuhr Coradazzi1; Lucas Marciel Soares Silva1; Thiago José Penachim2 |
|
|
Keywords: Peritonitis; Peritoneal fibrosis; Sclerosing encapsulating peritonitis; Computed tomography. |
|
|
Abstract: INTRODUCTION
Sclerosing encapsulating peritonitis (SEP) is a rare and curious entity of unknown etiology. Such a condition is characterized by partial or total encasement of the small bowel by a thick membrane of fibrotic connective tissue resembling a cocoon, that may extend and involve other organs such as the large bowel, liver and stomach(1). SEP presents with nonspecific clinical manifestations such as insidious abdominal pain and weight loss in addition to recurrent episodes of acute or subacute intestinal obstruction, either with or without the presence of an associated abdominal mass(2,3). This condition has been described with different names including "abdominal cocoon" by Foo in 1978(3), "peritonitis chronica fibrosa incapsulata" by Owtschinnikow in 1907(4), and "sclerosing encapsulating peritonitis" by Deeb et al. in 1998(4), many times in association with conditions which lead to recurrent peritonitis and peritoneal dialysis. The present report describes a case of preoperative tomographic diagnosis of SEP that was later confirmed by means of exploratory laparotomy, with emphasis on some clinical and imaging signs which contribute to increase the suspicion level, allowing for the planning of appropriate management and avoiding unnecessary surgical approach. CASE REPORT A male, brown, 37-year-old patient was referred by other service, complaining of diffuse abdominal pain and palpable abdominal mass for about one year, with probable diagnosis of umbilical hernia. The patient reported a previous history of paracoccidioidomycosis diagnosed for about 20 years ago, and over this period the condition progressed with voluminous ascites. Also, the patient reported a history of smoking and hepatic cirrhosis secondary to alcohol use with portal hypertension. At admission, clinical examination demonstrated a good general condition, with abdominal distension and presence of hydroaerial noise, besides a large, palpable, mobile and painful mass in the right hypochondrium and a small-sized umbilical hernia. Abdominal computed tomography (CT) demonstrated agglomerated small bowel loops with moderate distension at the level of the mesogastrium involved by a thick and regular membrane and a moderate amount of loculated ascitic fluid in the pelvis (Figure 1). 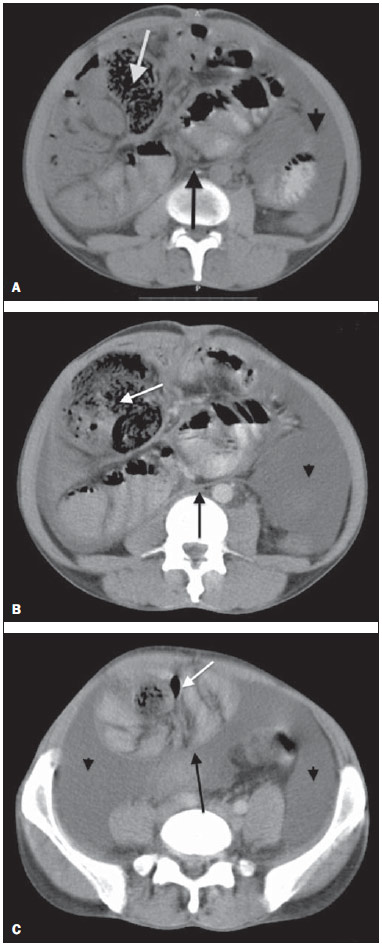 Figure 1. Non-contrast-enhanced (A) and contrast-enhanced (B,C) abdominal CT, axial sections, identifying conglomerate of small bowel loops with moderate air-fluid distension (white arrow) concentrated principally in the region of the mesogastrium and involved by a thick and regular membrane (black arrow), in association with thickening and greater enhancement of peritoneal reflections, as well as moderate amount of fluid with loculated aspect (arrow head) most concentrated in the pelvis. At the third day after the admission, the patient presented obstructive acute abdomen and was submitted to exploratory laparotomy. A thick, fibrous, grayish-white membrane was intraoperatively observed, involving small bowel loops in continuation to the visceral peritoneum, similar to a cocoon (Figure 2). Removal of the capsule and adhesionlysis were performed in addition to segmental enterectomy. 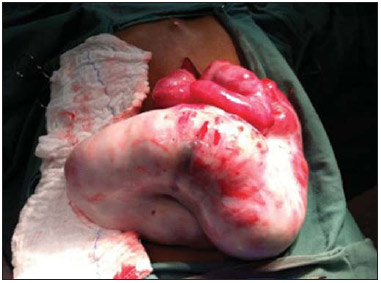 Figure 2. Surgical specimen. Intraoperative image of exploratory laparatomy showing a consistent abdominal mass with a thick, greyish-white membrane involving the small bowel loops, in continuation to the visceral peritoneum, resembling a cocoon. Histological analysis of the membrane that involved the small bowel loops revealed the presence of a chronic nongranulomatous inflammatory process in association with interstitial fibrosis, with vascular ectasia on a dense connective tissue, besides ischemic necrosis and acute serositis of the resected segment of small bowel. The patient's evolution was unsatisfactory, with diagnosis of entero-enteral fistula and at the 17th postoperative day further surgical procedure was required for enterocutaneous fistulotomy. After the procedure, the patient evolved with recurrent infections, severe denutrition and death 46 days after admission. DISCUSSION SEP may be classified into primary or idiopathic and secondary(1–4). Primary SEP has already been associated with retrograde menstruation in women and with abnormality in the embryonic development of the peritoneum, with possibility of concomitant greater omentum hypoplasia and mesenteric vessels malformation(1,3). Secondary SEP is associated with predisposing factors such as peritoneal dialysis, recurrent peritonitis, infectious and noninfectious granulomatous diseases, autoimmune diseases (systemic lupus erythematosus), long term practolol therapy, abdominal catheters (Le Veen shunts), intraperitoneal chemotherapy, liver transplant, cirrhosis, endometriosis, ovarian luteinized thecoma, S-protein deficiency, dermoid cyst rupture, exposure to asbestos and to fibrogenic materials(1). Almost all the cases of SEP described in the literature were intraoperatively diagnosed. A preoperative diagnosis requires a high level of clinical suspicion. Usually, the first clinical signs are nonspecific and frequently the condition cannot be recognized until the patient develops partial or total small bowel obstruction. Symptoms include pain and recurrent abdominal distension, nausea, vomiting, anorexia, weight loss, denutrition, recurrent episodes of acute, subacute or chronic intestinal obstruction, besides abdominal mass(1,4). Considering the nonspecificity of clinical findings of SEP, imaging methods become a useful tool for an early diagnosis, directly contributing in de adoption of an appropriate treatment. In patients with SEP, abdominal CT demonstrates agglomerated and distended small bowel loops concentrated in an abdominal segment, involved by a thick membrane, with peritoneal thickening, ascites and loculated fluid collections and possible peritoneal calcifications. Additionally, fibrosis leads to retraction of the mesenteric root, causing adhesions and loops conglomerate, leading to intestinal obstruction and dysfunction. Therefore, as compared with other imaging techniques, CT provides a comprehensive view of the condition as well as of any associated complication, besides helping to rule out other possible causes of intestinal obstruction(5). The treatment for SEP consists in surgical excision of the fibrotic membrane, intestinal loops adhesionlysis and resection in case of inviability of the affected intestinal segment. After appropriate surgical management, the prognosis is good, but it depends on the coexistence or not with other diseases(6). Finally, the present report highlights SEP as a rare disease, whose preoperative diagnosis depends on imaging evaluation, hence the great relevance of CT. Considering the relevance of the preoperative imaging diagnosis of SEP, it is necessary for the radiologist to be aware and attentive to the tomographic findings suggestive of the diagnosis. REFERENCES 1. Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol. 2012;18:1999–2004. 2. Hosein HH, Quane LK, Cohen AJ. Abdominal cocoon. Appl Radiol. 2003;32(10). 3. Altinli E, Sumer A, Celik A. Abdominal cocoon: a rare cause of intestinal obstruction. Israeli Journal of Emergency Medicine. 2007;7:42–4. 4. Ranganathan S, Abdullah BJJ, Sivanesaratnam V. Abdominal cocoon syndrome. J HK Coll Radiol. 2003;6:201–3. 5. Gupta S, Shirahatti RG, Anand J. CT findings of an abdominal cocoon. AJR Am J Roentgenol. 2004;183:1658–60. 6. Da Luz MM, Barral SM, Barral CM, et al. Idiopathic encapsulating peritonitis: report of two cases. Surg Today. 2011;41:1644–8. 1. MDs, Residents, Hospital e Maternidade Celso Pierro – Pontifícia Universidade Católica de Campinas (PUC-Campinas), Campinas, SP, Brazil 2. MD, Radiologist, Chief of Hospital e Maternidade Celso Pierro – Pontifícia Universidade Católica de Campinas (PUC-Campinas), Campinas, SP, Brazil Mailing Address: Dr. Vinicius de Barros Fernandes Avenida Onze de Junho, 970, ap. 71, Vila Clementino São Paulo, SP, Brazil, 04041-003 E-mail: vinicius.barros.fernandes@gmail.com Received April 19, 2013. Accepted after revision October 17, 2013. Study developed at Hospital e Maternidade Celso Pierro – Pontifícia Universidade Católica de Campinas (PUC-Campinas), Campinas, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554