Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 4 - July / Aug. of 2014
Vol. 47 nº 4 - July / Aug. of 2014
|
CASE REPORT
|
|
Lymphocytic mastopathy mimicking breast malignancy: a case report |
|
|
Autho(rs): Gabriela Couto Possati Campos1; Melissa Vieira Koch e Castro2; Viviane Ferreira Esteves de Mattos3; Laura Zaiden Ferreira e Pinto4; Marcia Cristina Bastos Boechat5; Alair Augusto Sarmet Moreira Damas dos Santos6 |
|
|
Keywords: Lymphocytic mastopathy; Breast cancer; Benign breast tumors. |
|
|
Abstract: INTRODUCTION
Lymphocytic mastopathy, also named fibrotic mastopathy, diabetic mastopathy, or sclerosing lymphocytic lobulitis, is a benign clinicopathological entity whose main differential diagnosis is breast carcinoma, affecting both young and middle-aged women (34–47 years)(1). The reported cases suggest an autoimmune etiology due to the association with clinical signs of insulin-dependent diabetes mellitus e its complications, particularly retinopathy, as well as other autoimmune diseases such as Hashimoto's thyreoiditis. In the present report, the authors describe a case of a woman who presented with a hardened retroareolar mass in her left breast. Clinical evaluation, mammography and ultrasonography suggested breast carcinoma. However, immunohistochemical study revealed lymphocytic mastopathy. CASE REPORT A 49-year-old patient was referred to the clinic of mastology of Instituto Fernandes Figueira (IFF) because of a palpable mass in the retroareolar region of the left breast. The patient reported a tick bite on the periareolar region of her left breast for three years which evolved with itching and focal hardening. The previous history of the patient did not include any disease. According to her gynecological history, she had spontaneous menarche at 12, with regular menstrual periods; had utilized hormone contraceptive for tem years (G2P2A0); her first pregnancy had been at the age of 18, and breastfed her first child for five months and the second for three months. Her last menstruation occurred at 44. At physical examination, the right breast did not present any abnormality. In the left breast a periareolar, whitish lesion, hardened at palpation was observed, besides skin thickening. Ultrasonography, mammography (Figure 1) and laboratory tests (blood count; fasting glucose test; lipid profile test; hepatogram; hepatitis A, B and C virus serology; VDRL) were requested. 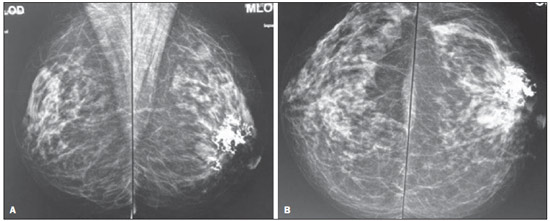 Figure 1. Mammography – right and left mediolateral oblique views (A) and right and left craniocaudal views (B). Homogeneously dense breasts containing an isodense, ovoid, well delimited nodule measuring about 20 mm in the left retroareolar region, in association with intermingled and adjacent extensive, coalescent and amorphous calcifications. The patient's blood count did not present any significant alteration; the requested serologies were negative; fasting glucose levels were increased (116 mg/dl); the hepatogram presented a subtle increase in TGO (37 U/l; reference value: up to 32 U/l), TGP (58 U/l; reference value: up to 31 U/l), GGT (41 U/L; reference value: up to 32 U/l); and no significant alteration was observed at the lipid profile test. Mammography demonstrated the presence of an isodense, ovoid, well delimited nodule measuring about 20 mm, located in the retroareolar region, in association with extensive, coalescent and intermingled and adjacent amorphous calcifications. Ultrasonography described extensive, ill-defined calcification in the retroareolar region, isoechoic nodule in the retroareolar region adjacent to the area of calcification, and an isoechoic nodule in the upper external quadrant of the left breast. The patient was referred to the IFF invasive procedures clinic. Core biopsy as performed in the region of the Gross calcifications and the histopathological results were suggestive of a nonspecific chronic inflammatory process. Correlation with immunohistochemical study was indicated for differential diagnosis with lymphoma. The immunohistochemical analysis indicated the diagnosis of lymphocytic mastopathy. DISCUSSION Lymphocytic mastopathy is a uncommon fibroinflammatory lesion that courses with the presence of benign breast nodules, and may clinically and radiologically mimic a carcinoma. Clinically, this lesion may appear as a single or multiple, uni- or bilateral, synchronous or asynchronous ill-defined, hardened mass(2). At clinical examination, mammography and ultrasonography, this lesion may mimic a breast carcinoma(3). In many cases, magnetic resonance imaging may define the lesion benignity(4,5). Magnetic resonance imaging is superior to mammography and ultrasonography in the differentiation between lymphocytic mastopathy and malignant lesions, and may be utilized as an appropriate guidance for the management of the benign lesions. The type of contrast enhancement may be a criterion to differentiate between lymphocytic mastopathy and malignant lesions. The contrast enhancement dynamics demonstrates a low, homogeneous, gradual and progressive uptake, without sudden washout. At fine needle aspiration biopsy, the sample has typically little or no cellular material, making the diagnosis more difficult(2). Ultrasonography-guided core biopsy can define the diagnosis in most cases, but in certain circumstances surgical biopsy is required(6,7). The histological diagnosis is characterized by dense, keloid-type fibrosis with lymphocytic ductitis and lobulitis, a perivascular lymphocytic infiltrate with breast lobules atrophy(8,9). Lymphocytic mastopathy pathogenesis is still to be elucidated and it is believed that it is multifactorial(10,11). There is association with long term type I diabetes mellitus, with poor management and multiple complications. This suggests that chronic hyperglycemia is involved in the development of the condition(12). Other factors must be involved in the pathogenesis of lymphocytic mastopathy, since its presence has been described in non diabetic patients or in those who did not receive insulin(13–15). Despite the alteration in her fasting glucose levels, the patient whose case is described in the present report does not have a diagnosis of diabetes mellitus and denied insulin therapy. CONCLUSION Breast carcinoma is the main differential diagnosis for lymphocytic mastopathy, therefore the knowledge about the clinical and radiological manifestations of this condition is of paramount importance. REFERENCES 1. Rosen PP. Inflammatory and reactive tumors In: Rosen PP, editor. Rosen's Breast pathology. New York: Lippincott-Raven; 1997. p. 46–9. 2. Logan WW, Hoffman NY. Diabetic fibrous breast disease. Radiology. 1989;172:667–70. 3. Mackey SP, Sinha S, Pusey J, et al. Breast carcinoma in diabetic mastopathy. Breast. 2005;14:392–8. 4. Wong KT, Tse GM, Yang WT. Ultrasound and MR imaging of diabetic mastopathy. Clin Radiol. 2002;57:730–5. 5. Yajima S, Fukutomi T, Akashi-Tanaka S, et al. Diabetic mastopathy: a case report with reference to the findings of enhanced computed tomography. Breast Cancer. 2001;8:246–9. 6. Sakuhara Y, Shinozaki T, Hozumi Y, et al. MR imaging of diabetic mastopathy. AJR Am J Roentgenol. 2002;179:1201–3. 7. Andrews-Tang D, Diamond AB, Rogers L, et al. Diabetic mastopathy: adjunctive use of ultrasound and utility of core biopsy in diagnosis. Breast J. 2000;6:183–8. 8. Tomaszewski JE, Brooks JS, Hicks D, et al. Diabetic mastopathy: a distinctive clinicopathologic entity. Hum Pathol. 1992;23:780–6. 9. Seidman JD, Schnaper LA, Phillips LE. Mastopathy in insulin-requiring diabetes mellitus. Hum Pathol. 1994;25:819–24. 10. Allen PW, Fisher C. Selected case from the Arkadi M. Rywlin International Pathology Slide Seminar: diabetic mastopathy. Adv Anat Pathol. 2001;8:298–301. 11. Camuto PM, Zetrenne E, Ponn T. Diabetic mastopathy: a report of 5 cases and a review of the literature. Arch Surg. 2000;135:1190–3. 12. Boullu S, Andrac L, Piana L, et al. Diabetic mastopathy, complication of type 1 diabetes mellitus: report of two cases and a review of the literature. Diabetes Metab. 1998;24:448–54. 13. Williams PH, Rubin CME, Theaker JM. Sclerosing lymphocytic lobulitis of the breast. Clin Radiol. 1995;50:165–7. 14. Ashton MA, Lefkowitz M, Tavassoli FA. Epithelioid stromal cells in lymphocytic mastitis – a source of confusion with invasive carcinoma. Mod Pathol. 1994;7:49–54. 15. Lammie GA, Bobrow LG, Staunton MD, et al. Sclerosing lymphocytic lobulitis of the breast – evidence for an autoimmune pathogenesis. Histopathology. 1991;19:13–20. 1. Trainee in Radiology and Imaging Diagnosis at Instituto de Pós-Graduação Médica Carlos Chagas (IPGMCC), Rio de Janeiro, RJ, Brazil 2. PhD, Assistant Professor, Pontifícia Universidade Católica do Rio de Janeiro (PUC-Rio), Radiologist, Instituto Fernandes Figueira (IFF), Rio de Janeiro, RJ, Brazil 3. PhD, MD, Unit of Mastology and Gynecology Clinic, Instituto Fernandes Figueira (IFF), Rio de Janeiro, RJ, Brazil 4. Master, MD, Mastologist, Instituto Fernandes Figueira (IFF), Rio de Janeiro, RJ, Brazil 5. PhD, Head of Radiology Service, Instituto Fernandes Figueira (IFF), Rio de Janeiro, RJ, Brazil 6. PhD, Full Professor, Course of Radiology and Imaging Diagnosis, Instituto de Pós-Graduação Médica Carlos Chagas (IPGMCC), Rio de Janeiro, RJ, Brazil Mailing Address: Dra. Gabriela Couto Possati Campos Rua Belfort Roxo, 266, ap. 903, Copacabana Rio de Janeiro, RJ, Brazil, 22020-010 E-mail: gabipossati@hotmail.com Received July 1, 2013. Accepted after revision October 17, 2013. Study developed at Instituto Fernandes Figueira (IFF) and at Instituto de Pós-Graduação Médica Carlos Chagas (IPGMCC), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554