Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 3 - May / June of 2014
Vol. 47 nº 3 - May / June of 2014
|
ICONOGRAPHIC ESSAY
|
|
Liliequist membrane: radiological evaluation, clinical and therapeutic implications |
|
|
Autho(rs): Daniel Aguiar Dias1; Fábio Luiz Onuki Castro2; James Henrique Yared3; Ademar Lucas Júnior4; Nelson Fortes Paes Diniz Ferreira5; Luiz Alves Ferreira Filho1 |
|
|
Keywords: Membrane; Liliequist; Third ventriculostomy; Magnetic resonance imaging; Neuroradiology. |
|
|
Abstract: INTRODUCTION
Liliequist membrane (LM) is a complex and variable structure. Initially described by Key and Retzius in 1875, it was further investigated by Liliequist in 1956 in his studies with pneumoencephalography in cadavers, and is well known by neurosurgeons. An increasing interest has been observed in relation to the study of Liliequist membrane, with the improvement and dissemination of minimally invasive endoscopic surgical techniques(1). In spite of being poorly recognized in the radiological community, the imaging evaluation of such a membrane is feasible in the greatest part of cases(1,2), and the knowledge about this structure is justified with a view on its several clinical and surgical implications, among them: a better preoperative planning; a possible failure of endoscopic ventricular shunt; and the clinical presentation of suprasellar arachnoid cysts and perimesencephalic hemorrhages(1,2). The relevance of a preoperative imaging evaluation of the LM is still to be established, in spite of the fact that studies report its feasibility, besides its positive influence on surgical outcomes(3). ANATOMY AND RADIOLOGICAL FINDINGS The LM can be identified as a thin structure (< 1 mm) with a thickness that is ever inferior to that of the tuber cinereum, located under the floor of the third ventricle, anteriorly extending from the dorsum sellae to the mammillary bodies. It may be considered a remnant of the primary tentorium, formed by either a single or double arachnoid layer and divided into three segments, as shown on Figures 1 and 2: the sellar, diencephalic and mesencephalic segments. The sellar segment is the most frequently seen on imaging methods, followed by the diencephalic and mesencephalic segments(1), and the latter two segments are also less frequently isolated in studies with cadavers(2). Such a finding can be explained by the fact that, as a rule, the mesencephalic segment is incomplete, thinner and presents a fenestration through which the basilar artery passes. 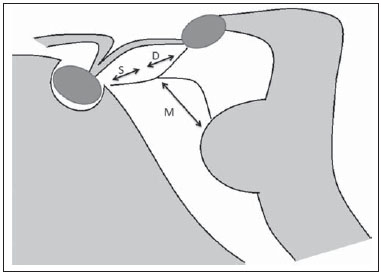 Figure 1. Schematic illustration of the LM anatomy, demonstrating the three segments of the Liliequist membrane in the sagittal plane. S, sellar segment; M, mesencephalic segment; D, diencephalic segment. (Modified by Fushimi et al.(1)). 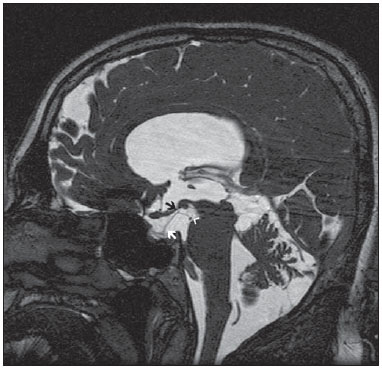 Figure 2. Radiological anatomy. Sagittal (slightly paramedian) CISS image clearly demonstrating the LM and its three segments. Note the membrane insertion into the mammillary body, and its thickness much inferior to that of the third ventricle floor. The white arrow identifies the sellar segment; the black arrow, the diencephalic segment; and the arrow head, the mesencephalic segment. In most cases reviewed in the literature, the membrane presents lateral insertions into the oculomotor nerves or adjacent to them, generally into the circumjacent arachnoid sheaths (Figure 3). The posterior anchoring is controversial, particularly in cases of retromammillary or premammillary insertion(1,2). 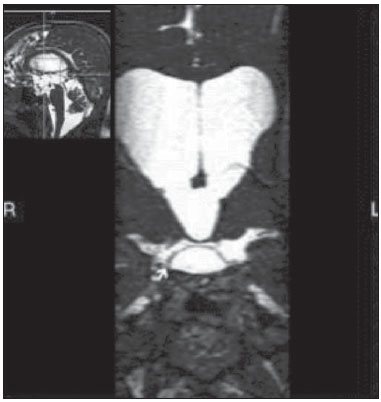 Figure 3. Radiological anatomy. CISS image with isotropic acquisition, which allows later multiplanar reformation, as above demonstrated in the coronal plane, highlighting lateral insertions of the LM adjacent to the sheaths of the third pairs of cranial nerves – an aspect that is more clearly demonstrated at right (arrow). Also, it is important to note that, principally because of its diencephalic component, the LM isolates the interpeduncular cistern from the chiasmatic cistern, with complete blockage in about 10-30% of cases. This fact is clinically relevant and, for this reason, it will be explored further. The thin membrane structure may be better evaluated by means of specific sequences with high contrast and anatomic resolution. For such a purpose, constructive interference in steady state (CISS) sequences, because of their cisternography effect, stands out as the best option, and is currently the imaging method of choice to evaluate cranial nerves, analyze cysts, cystic masses, neurocysticercosis and hydrocephalus. CISS is included in the family of fast gradient echo (GRE) sequences and presents different names according to the apparatus manufacturer: it is called fast imaging employing steady state acquisition (FIESTA) by General Electric, true fast imaging with steady-state precession (FISP) by Siemens, balanced fast field echo (FFE) by Philips, and true steady-state free precession (SSFP) by Toshiba(4). CLINICAL AND SURGICAL RELEVANCE Surgical implications Occlusion caused by the LM or even by other pre-pontine arachnoid trabeculae is already a well established cause of failure of endoscopic third-ventriculostomy, a surgical approach classically utilized for obstructive hydrocephalus resulting from aqueductal stenosis. It is important to note that, most recently, such a technique has also been utilized in some selected cases of non-obstructive hydrocephalus, with basis on new hydrodynamic concepts of the cerebrospinal fluid (CSF) flow, among them the case of relative aqueductal stenosis(5). In such a procedure, the neurosurgeon performs the puncture of the floor of the third ventricle, by direct visualization, communicating the third ventricle with the basal cisterns. Intraoperatively, the CSF flow can already be seen through the orifice. Also, in order to guarantee the success of the treatment, it is necessary to create a patent pathway from the interpeduncular and pre-pontine cisterns to the chiasmatic/suprasellar cistern. In case the flow between the cisterns is not visualized, one can suspect of imperforate LM, and fenestration may be performed in the same surgical session, if feasible. In such a context, it is important to note that a preoperative evaluation of the local anatomy, including an investigation and analysis of the arachnoid membranes could offer further information for the surgical planning. At the post-procedural imaging evaluation, one can visualize the CSF flow through the shunt, characterized by the presence of intense flow artifact (flow-void). In such a case flow contrast techniques (phase contrast) can be utilized, allowing for the analysis of the pulsatility of the cerebrospinal fluid flow, besides the possibility of measuring the stroke volume. It is important to note that the follow-up of ventricular dimensions do not represent a reliable parameter in the evaluation of the stomy patency. In case a dysfunctional the ventriculostomy orifice is evidenced either on the basis of clinical or imaging findings, an evaluation with 3D-CISS magnetic resonance imaging (MRI) may be extremely useful, since this method can differentiate between ventriculostomy orifice occlusion (generally by cicatricial tissue) and eventual mechanical obstruction by the LM and/or other arachnoid trabeculae in the prepontine cistern, according to the example illustrated on Figure 4(3,5,6). 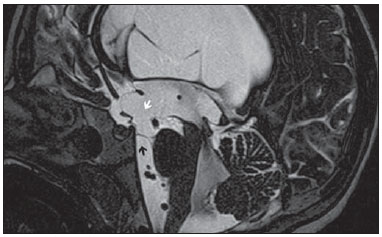 Figure 4. Failure of third-ventriculostomy. Postoperative image of endoscopic third-ventriculostomy. The presence of an intense flow artifact through the cerebral acqueduct toward the fourth ventricle is highlighted. Note the absence of flow identifiable by ventriculostomy orifice in the third ventricle, that is patent and wide (white arrow). Note the membrane in the pre-pontine cistern (black arrow) that may correspond to the lowered, intact Liliequist membrane or even pre-pontine membrane. Also, a remarkable global dilatation of the ventricular system is observed, probably resulting from obstruction at the level of the fourth ventricle output pathways where no sign of liquor cerebrospinalis flow is observed. Suprasellar arachnoid cysts Another condition whose genesis is intimately related to LM is suprasellar arachnoid cyst that may be understood as an imperforate LM invagination or diverticulum, with progressive accumulation of liquor cerebrospinalis. Thus, a cystic mass originates in the suprasellar cistern and may even herniate through the floor of the third ventricle and then towards the foramen of Monro, therefore causing obstructive hydrocephalus (Figure 5). 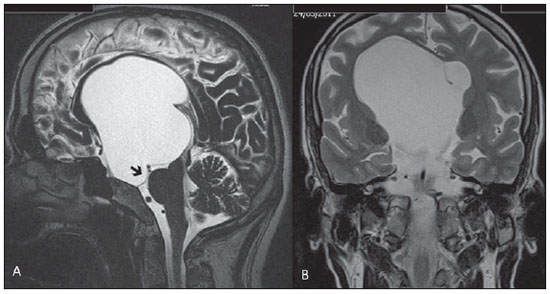 Figure 5. Suprasellar arachnoid cyst. Paramedian sagittal CISS image (A) and conventional, coronal, T2-weighted image (B). Note the presence of a large cystic mass insinuating from the suprasellar region toward the interior of the third and lateral right ventricle. On A it is possible to note the presence of the Liliequist membrane (arrow) forming the cystic wall. Besides the clinical condition of obstructive hydrocephalus, the symptoms are related to the typical mass effect of suprasellar lesions (visual disorders and endocrinological dysfunctions related to hypothalamic-hypophyseal axis dysfunction). Although this is a rare and nonspecific presentation, the classical bobble-head doll syndrome is described and many times may be erroneously interpreted as a tic in small children. The imaging findings may be the same as those of arachnoid cysts in other regions, characterized as thin walled cystic masses with a content isodense/isointense to the CSF on all the acquisitions and no enhancement after contrast medium injection. The absence of fat and hypersignal on diffusion weighted image (DWI) is useful in the eventual differentiation with dermoid and epidermoid cysts, respectively. The treatment of suprasellar cysts has advanced in the last years with the employment of minimally invasive endoscopic techniques, notwithstanding the controversy about the best procedure to be adopted (ventriculocystostomy versus ventriculocistocisternostomy)(7). Perimesencephalic hemorrhages Still, in relation to the relevant clinical implications, it is important to highlight the nonaneurysmal perimesencephalic hemorrhage. Some authors suggest that a more appropriate term for such a condition would be "nonaneurysmal pre-brainstem hemorrhage" since, generally, the bleeding epicenter is located anteriorly to the pre-pontine cistern. However, such bleedings may be seen at any point in the perimesencephalic cistern, as shown on Figure 6. 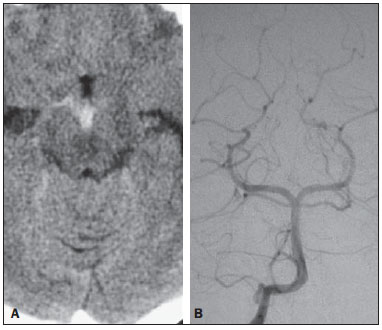 Figure 6. Perimesencephalic hemorrhage. Spontaneously hyperdense hematic material at the level of the interpeduncular cistern (non-contrast-enhanced computed tomography – image on A). Digital subtraction angiography (image on B) did not demonstrate any aneurysmal malformation (in this case, for illustrative purposes, demonstrating only the posterior circulation). In the case of such a condition, the previously described anatomical aspects acquire special significance, since they explain its physiopathology and diagnostic criteria. Perimesencephalic hemorrhage is a bleeding of uncertain etiology, but that is probably related to rupture of small perimesencephalic or capillary veins. In contrast to the more exuberant clinical signs observed in patients with aneurysm rupture and consequential subarachnoid hemorrhage, patients with nonaneurysmal perimesencephalic hemorrhage present with a milder ictus, marked by headache, meningism, photofobia and nausea, and, rarely, loss of consciousness. In principle, such a condition has a more favorable prognosis than subarachnoid bleedings secondary to aneurysm rupture, and complications such as rebleeding and vasospasm are less frequently observed. From the imaging diagnosis point of view, it is emphasized that, in general, the hematic material remains under the LM, i.e., in the perimesencephalic cistern (subdivided into interpeduncular, crural, ambient and quadrigeminal cisterns) and/or pre-pontine cistern. A significant amount of blood cranially flowing through the membrane towards the chiasmatic/suprasellar, Silvian or inter-hemispheric cisterns should be viewed with suspicion, i.e., the diagnostic possibility of aneurysmal rupture should be considered. Angiography is mandatory to rule out the presence of aneurysm, because about 3% of patients with basilar bifurcation aneurysm rupture meet the mentioned imaging criteria for nonaneurysmal perimesencephalic hemorrhage. The necessity of a second angiography is still discussed if an angiogram is negative, particularly in dubious cases or in high-risk patients(8). Therefore, the presence of a bleeding under the LM suggests the diagnosis of such a condition that has a distinctive physiopathological process and better prognosis than basal cistern hemorrhages secondary to aneurysmal rupture. CONCLUSION The knowledge about LM by radiologists is extremely relevant, either for a better understanding of the several related conditions, or even for an appropriate integration and dialogue with the requesting physician/surgeon. Furthermore, the recent advances of neurosurgical endoscopic techniques have renewed the enthusiasm for the study of the LM. Also, it is important to note that the introduction of high-detailing MRI techniques allows for a better evaluation and understanding of the anatomical particularities of such a structure. REFERENCES 1. Fushimi Y, Miki Y, Ueba T, et al. Liliequist membrane: three-dimensional constructive interference in steady state MR imaging. Radiology. 2003;229:360-5. 2. Froelich SC, Abdel Aziz KM, Cohen PD, et al. Microsurgical and endoscopic anatomy of Liliequist's membrane: a complex and variable structure of the basal cisterns. Neurosurgery. 2008;63(1 Suppl 1):ONS1-8. 3. Anýk I, Ceylan S, Koc K, et al. Membranous structures affecting the success of endoscopic third ventriculostomy in adult aqueductus sylvii stenosis. Minim Invasive Neurosurg. 2011;54:68-74. 4. Gonçalves FG, do Amaral LLF. Constructive interference in steady state imaging in the central nervous system. European Neurological Review. 2011;6:138-42. 5. Gangemi M, Maiuri F, Naddeo M, et al. Endoscopic third ventriculostomy in idiopathic normal pressure hydrocephalus: an Italian multicenter study. Neurosurgery. 2008;63:62-7. 6. Bargalló N, Olondo L, Garcia AI, et al. Functional analysis of third ventriculostomy patency by quantification of CSF stroke volume by using cine phase-contrast MR imaging. AJNR Am J Neuroradiol. 2005;26:2514-21. 7. El-Ghandour NM. Endoscopic treatment of suprasellar arachnoid cysts in children. J Neurosurg Pediatr. 2011;8:6-14. 8. Greenberg M. Handbook of neurosurgery. 7th ed. New York: Thieme; 2010. 1. MDs, Neuroradiology and Head & Neck Fellows at Hospital do Coração (HCor) and Teleimagem, São Paulo, SP, Brazil 2. MD, Neuroradiologist at Hospital do Coração (HCor) and Teleimagem, São Paulo, SP, Brazil 3. MD, Neuroradiologist at Hospital do Coração (HCor), Teleimagem, and Alta, São Paulo, SP, Brazil 4. MD, Neuroradiologist and Head & Neck Radiology, Hospital do Coração (HCor), Teleimagem, and Alta, São Paulo, SP, Brazil 5. MD, Head of the Neuroradiology Unit at Hospital do Coração (HCor), Teleimagem, and Alta, São Paulo, SP, Brazil Mailing Address: Dr. Daniel Aguiar Dias Rua Deputado Moreira da Rocha, 500, ap. 1600, Meireles Fortaleza, CE, Brazil, 60160-060 E-mail: daniel_aguiar@hotmail.com Received May 28, 2013. Accepted after revision September 9, 2013. Study developed at Hospital do Coração (HCor)-Associação do Sanatório Sírio e Teleimagem, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554