Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 1 - Jan. /Feb. of 2014
Vol. 47 nº 1 - Jan. /Feb. of 2014
|
ORIGINAL ARTICLE
|
|
A new method for intraoperative localization of epilepsy focus by means of a gamma probe |
|
|
Autho(rs): Omar Carneiro Filho1; Osvaldo Vilela Filho2; Paulo César Ragazzo3; Lea Mirian Barbosa da Fonseca4 |
|
|
Keywords: Radioguided surgery; Epilepsy; Gamma probe; Electrocorticography; Brain SPECT. |
|
|
Abstract: INTRODUCTION
Epilepsy is one of the most prevalent chronic neurological disorders, affecting 1-2% of the population worldwide(1,2). Notwithstanding the wide variety of currently available anticonvulsant drugs, 25-30% of patients remain refractory to the optimized conservative treatment. Such a refractoriness, that is generally greater in cases of partial epilepsy than in cases of generalized epilepsy, causes significant loss in quality of life and self-esteem, suggesting that, in such cases, the surgical treatment should be considered as a therapeutic alternative(3). Not all patients with refractory epilepsy, but only some of them, are candidates for surgery(4). Typically, patients with partial epilepsy are most frequently candidates for surgery than those with generalized epilepsy(5). The definition as regards surgical indication and surgical technique of choice depends on the location and number of epileptogenic foci (EFs), besides the type of epilepsy(6). In cases of generalized epilepsy, EFs are generally bilateral and multiple. Thus the foci resection is poorly feasible, and an option is made for either disconnective (corpus callosotomy) or neuromodulatory (left vagus nerve stimulation or deep cerebral stimulation of the ventromedial nucleus of thalamus) techniques. On the other hand, in cases of partial epilepsy (either simple or complex), the foci are unilateral and frequently located in a limited area of the brain. In such cases, option is made for EF resection. In cases where resection is unfeasible due to EF location in eloquent cerebral areas, one may opt for treatment with neuromodulatory techniques, such as deep brain stimulation of the anterior nucleus of the thalamus or hippocampus. Thus, one may conclude that the EF identification is a sine qua non condition for the surgical indication, requiring a careful diagnostic work-up to describe the convulsive seizure, including neuropsychological evaluation; electroencephalography (EEG); video EEG (for electroclinical correlation), either with or without invasive electrodes (foramen ovale, subdural or intracerebral); magnetic resonance imaging (MRI) to identify structural epileptogenic lesions, if applicable, by means of localization of temporal mesial sclerosis and small areas of cortical dysplasia; interictal or ictal single photon emission computed tomography (SPECT) to allow the identification of functionally abnormal areas corresponding to EFs. In case of resection surgeries, particularly in extratemporal epilepsy where invasive monitoring has not been employed, intraoperative confirmation of the EF is required. Electrocorticography is indicated in such cases and once the EF is identified, it is intraoperatively resected(6,7). The question that is proposed by the authors is the following: could an epileptogenic focus be interoperatively identified by any other mean? Cerebral perfusion scintigraphy or brain SPECT is a recognized complementary method for the diagnosis of a range of diseases. The intravenously administered radiotracer remains concentrated in the normal and abnormal areas of the cortex for many hours, differently from the other regions of the body which demonstrate a physiological washout. The idea of intraoperatively utilizing a portable gamma ray detector (gamma probe) is based on such a concept. The gamma probe is generally utilized to identify sentinel lymph nodes or primary lesions in cancer patients. The use of gamma probe in brain surgery that is known as gamma probe-assisted surgery was first introduced into the clinical practice to guide the resection of brain tumors(8). Considering that ictal brain SPECT can detect EFs and that the radiotracer remains in the abnormal cortical area for many hours, the authors have considered the hypothesis that the gamma probe might allow the intraoperative identification of the origination point of the epileptic seizures (Figure 1) . The present study was designed to test such hypothesis. 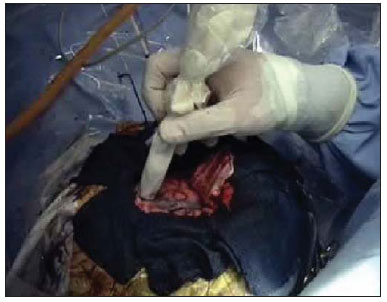 Figure 1. Gamma probe-assisted neurosurgery based on the more intense uptake quantified by gamma counting in the region of the epileptogenic focus. MATERIALS AND METHODS Two patients were enrolled in the present pilot study. Both patients presented with epilepsy refractory to optimized conservative treatment and signed a term of free and informed consent once they were informed about the objective of the present study - i.e. that the gamma probe would not be utilized to guide the surgery, but only to compare the results obtained with such instrument with those observed at electrocorticography. Brain SPECT scans were performed at the basal state (interictal phase) and during epileptic seizure (ictal phase), some hours before surgery. Besides the conventional dose, no additional radiopharmaceutical dose was required. All the radioguided surgical procedures were performed with the standard radiopharmaceutical dose applied some hours before the surgery. The nuclear medicine images (interictal and ictal phases) were presented to the neurosurgeons team immediately before the surgery. The other tests were performed on an inpatient basis. The patient 1 was a 25-year-old, right-handed man who presented with complex partial seizures (Five seizures per month on average) for 16 years. Interictal EEG demonstrated intermittent epileptogenic activity in the left temporal lobe, and ictal video-EEG showed the onset of the seizure in the left zygomatic and medial temporal electrodes. At MRI, a cavernoma was observed in the ipsilateral fusiform gyrus (Figure 2), and ictal SPECT demonstrated left temporal hyperperfusion (Figure 3). The neuropsychological evaluation revealed global worsening of his memory. 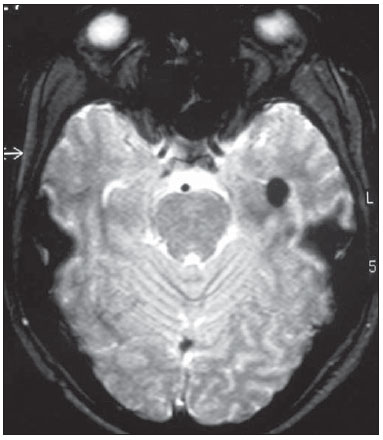 Figure 2. Patient 1 – magnetic resonance imaging demonstrating the presence of a cavernoma in fusiform gyrus of left temporal lobe.  Figure 3. Patient 1 – ictal brain SPECT scan demonstrating increase in the regional blood microcirculation of the left temporal lobe. The patient 2 was a 29-year-old, left-handed woman who presented with complex partial seizures and clonic seizures targeting the neck with rotation of the head to the left since her adolescence. Interictal EEG revealed epileptogenic activity in the T6, P4 and O2 electrodes. Ictal video-EEG demonstrated three typical seizures, but did not allow the identification or lateralization of the seizures origination point (inconclusive). MRI demonstrated posterior temporoparietal and right occipital atrophy (Figure 4), and ictal SPECT showed an extensive area of hyperperfusion surrounding a smaller area of hypoperfusion (Figure 5). The neuropsychological evaluation revealed only a slight impairment of the verbal memory. 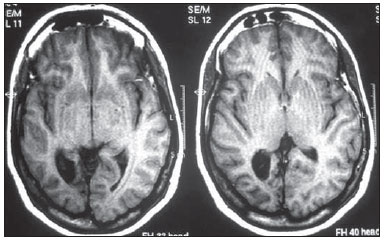 Figure 4. Cortical atrophy in the right brain hemisphere, specifically in the posterior temporoparietal areas and in the occipital lobe. 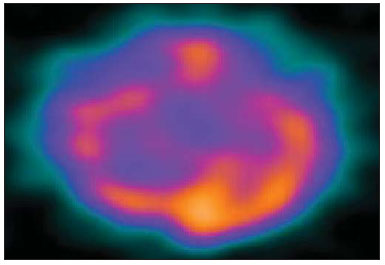 Figure 5. Patient 2 – interictal brain SPECT where a decrease in the cerebral blood microcirculation is observed in the posterior region of the right hemisphere in the posterior temporoparietal area and in the occipital lobe. The radiopharmaceutical utilized in the ictal SPECT scans was technetium-99m labeled ethylenediamine cysteine (EDC) manufactured by Instituto de Pesquisas Energéticas e Nucleares - Comissão Nacional de Energia Nuclear, Brazil. The patients underwent surgery few hours (less than five hours) after EDC injection (ictal phase). The surgery was performed under general anesthesia. After craniotomy on the probable epileptogenic area, radioactivity counting was performed with the gamma probe (Neoprobe 2000; Johnson & Johnson, USA). The areas with increased or decreased (patient 2) counts were marked with a cotton thread. Subsequently, electrocorticography was performed with subdural plate and depth electrodes implanted on the head of the hippocampus and amygdala, initially under spontaneous activity and subsequently after activation with alfentanyl. The left temporal hyperperfusion area (patient 1) was delineated and resected. Then, the mapped areas of epileptogenic activity (electrocorticography) were delineated and compared with those detected by the gamma probe. Once the lesionectomy was completed, a new radioactivity counting was performed at the resection site and on the surgical specimen (ex-vivo). RESULTS In the patient 1, the electrographic study of spontaneous activity revealed to be basically normal. After intraoperative activation with alfentanyl(9) electrocorticographic seizure onset was observed on the head of the hippocampus. Maximum radioactive count was initially observed at the middle temporal gyrus at about 3.5 cm posteriorly to the tip of the left temporal lobe. After corticotomy, the gamma probe indicated maximum count at the head of the hippocampus. Resection of the cavernoma and anteromedial temporal lobectomy were performed by the Spencer technique. In the patient 2, once the dura mater was opened, an area of atrophy and gliosis corresponding to one found at MRI was observed. Radioactivity counting confirmed the ictal SPECT findings, i.e. decreased radioactivity count in the atrophic gliosis area, and increased radioactivity count in superiorly, inferiorly and anteriorly located areas. The electrocorticographic study during spontaneous activity demonstrated a polyspike and slow-wave pattern in the atrophic gliosis area and, after activation with alfentanyl, almost continuous spikes in the same area, propagating towards the superior parietal lobe and, posteriorly, to the temporal lobe and head of the hippocampus(9). After lesionectomy, the adjacent areas showed normal radioactivity count. The congruence between electrocorticographic findings and radioactivity counts was practically similar in both patients. After a two-year clinical follow-up, the authors observed absence of epileptic seizures in both patients. DISCUSSION A significant number of intractable long-lasting epilepsies may be due to the presence of cerebral lesions which can be identified at neuroimaging studies, such as mesial temporal sclerosis, cortical dysplasias, benign tumors and cavernomas. Resection of such lesions is extremely relevant for a better management of convulsive seizures. Such lesions may be or not be visible to the naked eye, and their identification may require some preoperative or even intraoperative techniques, including morphological methods (CT and MRI), functional methods (SPECT, PET, EEG) or electrocorticography(10). Other intraoperative methods such as MRI and CT imply high costs and are available only in a few centers around the world(10). A feasible alternative would be the utilization of neuronavigation surgery(11), whose hardware can be rented. However, besides being restricted to few clinical centers, a problem inherent to neuronavigation is the intraoperative brain shift due to loss of cerebrospinal fluid (CSF) and intracranial invasion of subdural air after opening of the dura mater. Despite their efficacy in the identification of brain lesions, such methods cannot identify functionally abnormal areas. Not infrequently, the epileptogenic area is larger than the structurally abnormal area, as observed in patient 1, who had a cavernoma in the left fusiform gyrus whose EF was extended to the head of the hippocampus. At structural neuroimaging, some patients present with more than one lesion, and it is impossible to define which of such lesions would be the initial cause of the intractable epilepsy. Finally, it is important to highlight that many patients with intractable epilepsy do not present any evidence of structural lesion (cryptogenic epilepsy), and, in such cases, the contribution of MRI, CT and neuronavigation would be null(11). Studies and researches corroborate the fact that complex partial seizures correspond to approximately 55% of convulsive seizures in adult individuals. Most of such seizures originate in the temporal lobe and, in such cases the surgical treatment is made with standard techniques such as temporal lobectomy or selective amygdalohippocampectomy, so electrocorticography becomes virtually unnecessary(9). However, about 20% of the complex partial seizures have an extratemporal origin. In such situations, particularly when structural lesions are not demonstrated, the intraoperative electrocorticographic recording becomes mandatory for identification of the epileptogenic area. Such a method is considered as the gold standard for this purpose. In the present study, the authors have demonstrated that the radioactivity counting by means of a gamma probe was as effective as the intraoperative electrocorticographic recording in the detection of the epileptogenic area in both patients submitted to the technique. This technique did not require administration of any additional radiopharmaceutical dose. The dose injected for the ictal SPECT study is sufficient, provided the surgery is performed within the next few hours. The gamma probe can detect regions with greater radiotracer concentration by means of the sound and reading of radioactivity countings. Differently from gamma chambers which produce images, gamma probes provide digital counts. It operates like a unidirectional microphone, detecting gamma rays exclusively from structures located in front of it. The functionally abnormal epileptogenic area concentrates greater radiopharmaceutical activity in relation to the normal tissue, for about up to ten hours, and the gamma rays can be detected by the gamma probe. Thus, while electrocorticography identifies the epileptogenic area, the gamma probe identifies the functionally (or structurally) abnormal area which virtually correspond to each other. It is important to highlight that it is a safe method that does not interfere with the diagnostic dynamics at any moment, enabling a directed surgical approach. The major limitation of the present study is related to the low number of patients submitted to the technique. Further prospective, randomized studies with a much higher number of patients, comparing electrocorticography and gamma probe findings are required to confirm the present results. CONCLUSION The method presently described seems to be extremely useful in the detection of epileptogenic areas, since it demonstrates close correlation with electrocorticography findings (gold standard), with the advantage of noninvasiveness, demonstrating in loco the epileptogenic focus. Another additional advantage would be the capacity of demonstrating the decrease in radioactivity counts at the resection site after the removal of the epileptogenic area. The authors conclude that possible indications for the present technique would be for extratemporal epilepsy, in cases with more than one structural lesion (dual pathology), and in cases where MRI does not demonstrate structural alterations (cryptogenic epilepsy) and where electrocorticography might have a significant difficulty in the intraoperative detection of the epileptogenic focus. The study demonstrates that radioactivity counting by means of a gamma probe was as effective as the intraoperative electrocorticography to detect epileptogenic area in both patients. Thus the results obtained with both techniques were practically congruent. REFERENCES 1. Shorvon S. Status epilepticus: its clinical features and treatment in children and adults. Cambridge: Cambridge University Press; 1994. 2. Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46:470-2. 3. Blumer D. Psychiatric aspects of intractable epilepsy. Adv Exp Med Biol. 2002;497:133-47. 4. Carreño M, Lüders HO. General principles of presurgical evaluation. In: Lüders HO, editor. Textbook of epilepsy surgery. London, UK: Informa Healthcare; 2007. p. 407-22. 5. Roger J, Bureau M, Dravet C, et al. Epileptic syndromes in infancy, childhood, and adolescence. 4th ed. Montrouge, France: John Libbey Eurotext; 2005. 6. Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7:514-24. 7. Palmini A, Gambardella A, Andermann F, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476-87. 8. Vilela Filho O, Carneiro Filho O. Gamma probe-assisted brain tumor microsurgical resection. A new technique. Arq Neuropsiquiatr. 2002;60:1042-7. 9. McGuire G, El-Beheiry H, Manninen P, et al. Activation of electrocorticographic activity with remifentanil and alfentanil during neurosurgical excision of epileptogenic focus. Br J Anaesth. 2003;91:651-5. 10. von Oertzen TJ, Mormann F, Urbach H, et al. Prospective use of subtraction ictal SPECT coregistered to MRI (SISCON) in presurgical evaluation of epilepsy. Epilepsia. 2011;52:2239-48. 11. Cho DY, Lee WY, Lee HC, et al. Application of neuronavigator coupled with an operative microscope and electrocorticography in epilepsy surgery. Surg Neurol. 2005;64:411-8. 1. Specialist in Nuclear Medicine, Director, Cebramen - Centro Brasileiro de Medicina Nuclear e Imagem Molecular and Imen - Instituto de Medicina Nuclear, Goiânia, GO, Brazil 2. PhD, Associate Professor and Head of the Unit of Neurosurgery, Hospital das Clínicas - School of Medicine, Universidade Federal de Goiás (UFG), Associate Professor at Pontifícia Universidade Católica de Goiás (PUC Goiás), Goiânia, GO, Brazil 3. PhD, Neurologist, Director of the Unit of Epilepsy and Neurophysiology, Instituto de Neurologia de Goiânia, Goiânia, GO, Brazil 4. PhD, Full Professor of Nuclear Medicine, Department of Radiology - Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil Mailing Address: Dr. Omar Carneiro Filho Alameda dos Buritis, 600, Centro Goiânia, GO, Brazil, 74015-080 E-mail: dromarcarneiro@gmail.com Received October 29, 2012. Accepted after revision August 23, 2013. Study developed at Instituto de Neurologia de Goiânia, Imen - Instituto de Medicina Nuclear and at Cebramen - Centro Brasileiro de Medicina Nuclear e Imagem Molecular, Goiânia, GO, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554