Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 1 - Jan. /Feb. of 2014
Vol. 47 nº 1 - Jan. /Feb. of 2014
|
ORIGINAL ARTICLE
|
|
Prediction of preeclampsia by means of Doppler flowmetry of uterine artery and flow-mediated dilation of brachial artery |
|
|
Autho(rs): Aline Costa Calixto1; Augusto Henriques Fulgêncio Brandão2; Luana Lopes Toledo1; Henrique Vítor Leite3; Antônio Carlos Vieira Cabral4 |
|
|
Keywords: Preeclampsia; Doppler flowmetry; Vascular endothelium; Prediction. |
|
|
Abstract: INTRODUCTION
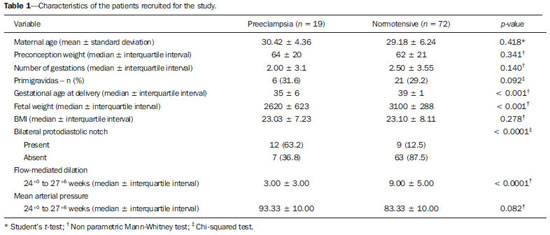
Pregnancy-specific hypertensive disorders are responsible for the greatest part of maternal mortality and perinatal morbidity worldwide. Preeclampsia (PE) stands out in this scenario, increasing the risk for events such as premature placental separation, acute renal failure, cerebral hemorrhage, hepatic insufficiency, pulmonary edema and disseminated intravascular coagulation, besides possible progression to severe presentations such as eclampsia and HELLP syndrome. In all over the world, 10-15% of maternal deaths are directly related to PE or to eclampsia(1). The morbimortality rate is also significant among fetuses and neonates as a function of the increased risk of restricted intrauterine growth and preterm delivery in cases of pregnancies affected by PE. Despite the clinical and social relevance of such condition, even nowadays its prediction represents a challenge and therefore a fertile area for studies. PE is a multifactorial condition characterized by increase in the arterial pressure associated with proteinuria whose clinical manifestation typically occurs in the second half of the gestation. The association between PE and problems in the development of the placental vascularization at early stages of pregnancy is known e documented(2,3). Such alterations may result in placental hypoperfusion and thus cause hypoxia and ischemia, leading to increase in the production of antiangiogenic factors and other substances capable of causing systemic endothelial dysfunction. An injured endothelium is characterized by increased vascular permeability, vasoconstriction, activation of the coagulation system and microangiopathic hemolysis. All those alterations are responsible for hypertension, proteinuria and other clinical manifestations of PE such as visual disorders, headache, epygastralgia, thrombocytopenia and hepatic impairment. The systemic endothelial dysfunction caused by the chain of events initiated with inappropriate placentation explains the clinical manifestations of PE(4). Arterial pressure elevation results from vascular wall tonus control deregulation. Proteinuria and edema are caused by the increase in the vascular permeability. Coagulopathy is the result from abnormal endothelial expression of coagulation factors. Headache and convulsion, visual symptoms, epygastralgia and fetal growth restriction are sequelae from vascular endothelial dysfunction of target organs - brain, liver, kidneys and placenta. Laboratory evidences which corroborate the relationship between systemic endothelial dysfunction and PE include serum fibronectin concentration, factor VIII and thrombomodulin(5); impaired flow-mediated vasodilatation(7); decreased production of vasodilators such as nitric oxide and prostacyclin; increased production of vasoconstrictors such as endothelins and thromboxanes; and, biophysically, by impairment of the brachial artery flow-mediated dilation (FMD)(8,9). Once the physiopathology of PE is briefly understood, the poor placental perfusion and endothelial dysfunction may be perceived as the most remarkable events in the development of such a condition. Considering that both events precede the clinical manifestations of the disease, and taking the mentioned evidences into consideration, it is suggested that clinical methods aimed at evaluating these two events may be useful to predict PE. The present study was aimed at evaluating the association of Doppler flowmetry of uterine artery and FMD as methods to predict PE. As already mentioned, the tests evaluate, respectively, the placental perfusion and the endothelial function which represent the determining factors in the PE physiopathology events preceding the clinical manifestations of the disease. MATERIALS AND METHODS Patients A total of 91 patients were recruited for the present longitudinal study at the unit of high-risk prenatal assistance of Hospital das Clínicas da Universidade Federal de Minas Gerais (HC-UFMG). The study was approved by the Committee for Ethics in Research of HC-UFMG. After undergoing the regular prenatal consultation between 24+0 and 27+6 weeks of gestation, the patients were invited to participate in the present study. The selected patients were given information about the study at the moment of their recruitment and subsequently signed a term of free and informed consent. Then, the patients were submitted to Doppler flowmetry of uterine arteries and FMD. All the procedures were performed by a single trained and certified sonographist of the HC-UFMG. Amongst the 91 patients, 19 pregnancies were complicated by PE and the other 72 patients were not diagnosed with the condition until two weeks after delivery. All the selected patients presented with at least one of the following risk factors for development of PE, according the study by Duckitt et al.(10): chronic arterial hypertension (23; 25.5%); pregestational diabetes mellitus (according to the criteria defined in 2011 by the American Diabetes Association(11)) (16; 17.5%); personal history of PE in a previous gestation (21; 23.0%); familial history (either mother or sister) of PE (16; 17.5%); high body mass index (defined as > 35 kg/m2) (15; 16.5%). The criteria for defining the presence of chronic arterial hypertension were the following: patient diagnosed with hypertension before the pregnancy; patient with pressoric levels > 140 × 90 mmHg before the 20th gestational week; or patient who remained hypertensive for at least 12 weeks after delivery. The diagnosis of PE was made according to the criteria defined by the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy, 2000(12). According to such a classification, PE is defined as elevation of the arterial pressure after 20 gestational weeks pressoric levels > 140 × 90 mmHg observed at two measurements with a six-hour interval), associated with the presence of proteinuria (1+ or above at proteinuria tape or 24h proteinuria > 0.3 g/24 h). The superimposition of PE in patients with chronic arterial hypertension was considered, according to the American College of Obstetricians and Gynecologists report(13) (which was modified by the authors' institution), when one of the following factors was present: 1) marked arterial pressure elevation (> 160 × 110 mmHg); 2) massive proteinuria (> 2.0 grams in 24 hours); 3) significant increase in pressoric levels after a period of appropriate management; 4) serum creatinine levels > 1.2 mg/dl. Methods Flow-mediated dilation of brachial artery The FMD technique was performed with a SonoAce 8800 color Doppler ultrasonography apparatus (Medison; Seoul, South Korea) with a 4-8 MHz linear transducer. The patients were placed at rest for 15 minutes in dorsal decubitus. All the patients had their arterial pressure measured and their brachial artery medially identified in the antecubital fossa of the dominant upper limb. An image from the vessel was obtained at approximately 5 cm from the upper limb elbow, and a longitudinal section (B-mode) was performed during the moment of lesser distension of the vessel, corresponding to the cardiac diastole. The image was obtained by means of image recovery on the cine loop display of the equipment and frozen to get a mean of the three measurements of the vessel caliber (D1). After this first measurement, the sphygmomanometer cuff positioned distally (over the forearm) to the site of the brachial artery measurement was inflated for five minutes up to a pressure > 250 mmHg, and later was slowly deflated, The mean of three further measurements of the vessel caliber was obtained with the already described technique one minute after the cuff deflation (D2), The FMD value was obtained by the following equation: FMD (%) = [(D2 - D1)/D1] × 100 where: D1 = basal diameter and D2 = post-occlusion diameter. Doppler flowmetry of uterine arteries Doppler flowmetry of uterine arteries is performed with a 3.5 MHz convex transducer. The artery insonation is performed at its proximal third at a maximum 60º angle. The evaluation of the uterine arteries flow is based on a wave similar to at least three other symmetrical waves. The presence of the protodiastolic notch was the parameter considered on the flow wave, and the study was considered as altered only in the cases where the incisures was present bilaterally. Statistical analysis The continuous data normality was analyzed with the Shapiro-Wilks test. The Student's t-test was utilized to compare variables with normal distribution between groups of patients who developed PE. The Pearson's chi-squared test was utilized to compare categorical variables and the Student's t test to compare continuous variables without normal distribution. Statistical significance was defined with p < 0.05. The analyses were performed with the software SPSS®19 (SPSS Inc.; Chicago, IL, USA). Sensitivity and specificity were calculated isolatedly or associated in parallel. The FMD cutoff value was defined with basis on a study developed in the authors' institution with a population considered at high-risk for developing PE. RESULTS Amongst the 91 patients, 19 developed PE and 72 remained normotensive during the whole gestational period, delivery and puerperium. Table 1 presents demographic and gestational data, as well as the analysis of the sonographic studies of the patients who developed PE and of the group without PE. Doppler flowmetry of uterine arteries was considered as altered in cases where the presence of protodiastolic incisures was observed bilaterally. The FMD study was considered as altered in the cases where the percentage of brachial artery dilation after induced hyperemia was < 6.5%. Table 2 presents the values for sensitivity and specificity of the techniques in the prediction of PE, either isolatedly or associated in parallel analysis. 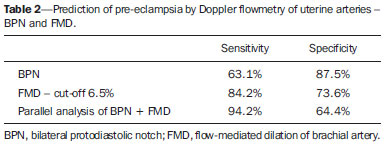 DISCUSSION The identification of pregnant women at increased risk for development of PE may determine a more specialized and rigorous prenatal follow-up, allow earlier interventions as necessary and thus predict and even change the PE natural history, improving maternal and perinatal outcomes related to the condition(2,14). As regards the analysis of the demographic and gestational data as determining factors in the prediction of PE, only gestational age at the delivery, fetal weight, presence of bilateral protodiastolic notch at Doppler flowmetry of uterine arteries and presence of alterations at FMD demonstrated statistically significant difference between the group of normotensive patients and the group of patients who developed PE. The analysis of results demonstrates an increment in sensitivity (94.2%) to predict PE in cases of association in parallel between the identification of presence of bilateral protodiastolic notch and altered FMD values, as compared with their evaluation as isolated prediction factors (63.1% and 84.2%, respectively). Endothelial injury evaluated by FMD is well established in patients with diagnosis of PE, either in cases of early or late presentation(15). In a study developed by Savvidou et al., the methods association was more effective in the identification of the patients at risk for developing PE and restricted intrauterine growth than Doppler flowmetry of uterine arteries alone(16). As regards FMD, its utilization alone late in the second gestational trimester could identify approximately 90% of cases of PE, in a study developed by Takase et al.(17). The FMD results are significantly poorer in patients with late development of PE as from the 24th gestational week(18,19). The association between the indirect evaluation of the endothelial function by FMD and of the placental perfusion by Doppler flowmetry of uterine arteries has proved to be highly accurate in the prediction of PE. In this context, the authors envisage further studies to deepen the direct evaluation of the endothelial function with biochemical markers such as asymmetric dimethylarginine, angiogenesis factor and vasoactive peptides. This would allow for the identification of a group that could benefit from more invasive investigations such as angiotensin II test or even therapies still under study such as dietary supplementation with L-arginine, or the administration of angiotensin 1-7. Additionally, FMD might be utilized to evaluate the response to the introduction of such pharmaceuticals. One might raise the theoretical hypothesis that in the case of reversion of an established endothelial injury, a concomitant decrease in the risk for developing PE might occur. REFERENCES 1. World Health Organization. The World Health Report 2005 - make every mother and child count. Geneva: World Health Organization; 2005. 2. Barton JR, Sibai BM. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;112(2 Pt 1):359-72. 3. Magee LA, Helewa M, Moutquin JM, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3 Suppl):S1-48. 4. Pennington KA, Schlitt JM, Jackson DL, et al. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5:9-18. 5. Lyall F, Greer IA. The vascular endothelium in normal pregnancy and pre-eclampsia. Rev Reprod. 1996;1:107-16. 6. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243-9. 7. Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173-92. 8. Savvidou MD, Kametas NA, Donald AE, et al. Non-invasive assessment of endothelial function in normal pregnancy. Ultrasound Obstet Gynecol. 2000;15:502-7. 9. Castro PT, Montenegro CAB, Carvalho ACP, et al. Dilatação fluxomediada da artéria braquial em mulheres com artrite reumatóide. Radiol Bras. 2007;40:247-50. 10. Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. 11. American Diabetes Association. Standards of medical care in diabetes - 2011. Diabetes Care. 2011;34 Suppl 1:S11-61. 12. [No authors listed]. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22. 13. ACOG Committee on Practice Bulletins - Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159-67. 14. Plasencia W, Maiz N, Poon L, et al. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks and 21 + 0 to 24 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:138-46. 15. Brandão AHF, Barbosa AS, Lopes APBM, et al. Dopplerfluxometria de artérias oftálmicas e avaliação da função endotelial nas formas precoce e tardia da pré-eclâmpsia. Radiol Bras. 2012;45:20-3. 16. Savvidou MD, Hingorani AD, Tsikas D, et al. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361:1511-7. 17. Takase B, Goto T, Hamabe A, et al. Flow-mediated dilation in brachial artery in the second half of pregnancy and prediction of pre-eclampsia. J Hum Hypertens. 2003;17:697-704. 18. Brandão AH, Cabral MA, Leite HV, et al. Endothelial function, uterine perfusion and central flow in pregnancies complicated by preeclampsia. Arq Bras Cardiol. 2012;99:931-5. 19. Brandão AH, Pereira LM, Gonçalves AC, et al. Comparative study of endothelial function and uterine artery doppler velocimetry between pregnant women with or without preeclampsia development. J Pregnancy. 2012;2012:909315. 1. MDs, Gynecologists and Obstetricians, Hospital das Clínicas da Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 2. PhD, MD, Gynecologist and Obstetrician, Hospital das Clínicas da Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 3. PhD, Associate Professor, Department of Gynecology and Obstetrics, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 4. PhD, Full Professor, Department of Gynecology and Obstetrics, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil Mailing Address: Dr. Augusto Henriques Fulgêncio Brandão Universidade Federal de Minas Gerais (UFMG) - Maternidade Otto Cirne-HC Avenida Professor Alfredo Balena, 110, 4º andar, Santa Efigênia Belo Horizonte, MG, Brazil, 30130-100 E-mail: augustohfbrandao@hotmail.com Received April 7, 2013. Accepted after revision September 2, 2013. Financial support: Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig). Study developed at Hospital das Clínicas da Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554