Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 4 - July / Aug. of 2013
Vol. 46 nº 4 - July / Aug. of 2013
|
ICONOGRAPHIC ESSAY
|
|
Mucinous carcinoma of the breast: iconographic essay with histopathological correlation |
|
|
Autho(rs): Gustavo Nunes Medina Coeli1; Henrique Ferreira dos Reis1; Dayse Ribeiro Bertinetti1; Francesca Maia Faria2; Daniel Guimarães Tiezzi3; Tatiane Mendes Gonçalves de Oliveira4 |
|
|
Keywords: Breast neoplasm; Mucinous carcinoma; Mammography; Ultrasonography; Magnetic resonance imaging. |
|
|
Abstract: INTRODUCTION
Breast cancer is the most common malignant tumor in women. The Brazilian radiological literature has emphazised the importance of imaging methods in the improvement of breast cancer diagnosis(1–4). Mucinous or colloid breast carcinoma (MBC) is an uncommon subtype of invasive ductal carcinoma (IDC), representing between 1% and 7% of all breast neoplasms. This tumor shows a wide age distribution, but with higher incidence in elderly(5). Histologically, such variant is characterized by a tumor-like arrangement of neoplastic cells involved by extracellular mucin, in most cases associated with peripheral ductal carcinoma in situ (DCIS)(6). Cellularity as well as the amount of mucin has large variation among mucinous tumors. The greater the amount of mucin, the better the prognosis is(6). There are two histological presentations with different imaging features and prognoses: pure and mixed types. Pure type comprises tumors with a mucinous arrangement in their whole extent, with less than 10% of non-mucinous component, or even a poorly differentiated non-mucinous component(6,7). The mixed type presents a greater amount of neoplastic cells not involved by mucin, generally in association with a smaller amount of extracellular mucin, which implies intermediate characteristics between the pure type and nonspecific invasive ductal carcinoma (NS-IDC), for this reason sometimes referred to as "IDC with mucinous differentiation"(6,7). The present pictorial essay focus on the most typical imaging findings of such specific type of carcinoma and its subtypes, highlighting findings associated with prognostic prediction. Histological diagnosis The diagnosis of mucinous neoplasm may be suspected at fine-needle aspiration biopsy (FNAB), although with low accuracy in the differentiation between benign (mucoceles) and malignant mucinous lesions (MMC). Core biopsy exhibits high sensitivity and high positive predictive value (PPV) for the diagnosis of mucinous neoplasm, particularly in cases where such biopsy is imaging-guided, reaching values close to 100%(8,9). However, the differentiation between pure and mixed types can only be established after excision and evaluation of the lesion in its whole extent(6,9). Prognosis The better prognosis of MMCs, compared with NS-IDC, explains the diagnostic importance of these tumors(7). Pure type shows indolent growth, while the mixed type presents variable biological behavior, sometimes similar to NS-IDCs(10). Thus, MMCs, specially the pure type, demonstrated a lower histological degree (well differentiated tumors), higher hormone receptor (HR) expression, lower incidence of adverse oncogenes, lower rate of axillary lymph node involvement at diagnosis, and longer disease-free survival (with no significant difference in overall survival)(7,11,12). Imaging findings MMC is part of a group of 10% to 20% of the malignant tumors which present as circumscribed lesions, sometimes initially interpreted as benign lesions, which delays the diagnosis and appropriate treatment(5,8,12) decisions. In such a context, mammography, ultrasonography and magnetic resonance imaging represent fundamental tools in the attempt to recognize findings not only suggesting the diagnosis of MMC, but also helping to differentiate between the two histological types of the neoplasm directly related to different prognostic factors(11,13–15). Typically, a MMC presents as an ovoid or round nodule with circumscribed margins. At mammography, the pure type correlates with circumscribed or micro-lobulated margins, which present a direct relationship with the amount of extracellular mucin(16), as demonstrated on Figure 1. The mixed type presents more indistinct or spiculated contours secondary to a higher degree of fibrosis and peripheral desmoplasia, similarly to a NS-IDC. Microcalcifications are not common, and when they occur, rarely represent psammomatous calcifications, most times associated with the presence of peripheral component of DCIS(6,16,17). 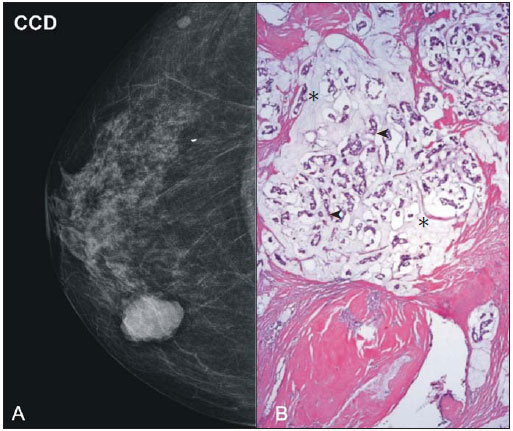 Figure 1. A: Female, 69-year-old patient. Craniocaudal view demonstrating nodule with microlobulated margins in the medial quadrant of the right breast, diagnosed as pure MMC. B: Histological section of the lesion, identifying mucinous tumor with neoplastic cells involved by moderate amount of extracellular mucin. Ultrasonography has higher sensitivity than mammography in the detection of MMCs(16,18). Characteristically, MMCs are visualized as an ovoid nodule with circumscribed or microlobulated margins, and may be either hypo- or isoechoic, with posterior acoustic shadowing in 37% to 71% of the cases, as demonstrated on Figure 2. 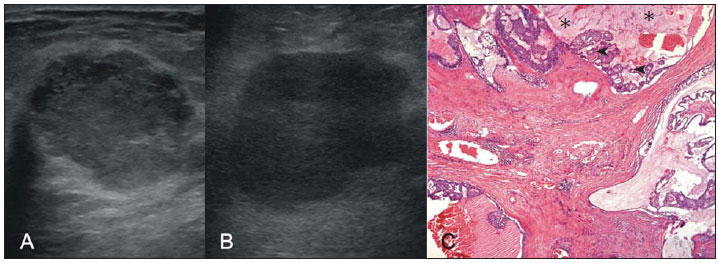 Figure 2. A: Female, 69-year-old patient with pure MMC, with typical sonographic findings: ovoid and microlobulated nodule, isoechoic to fat tissue and with posterior acoustic enhancement. B: Female, 58-year-old patient with an ovoid nodule with indistinct and angulated margins, with no noticeable microlobulation, with posterior acoustic enhancement, diagnosed as mixed MMC. C: Histology from this patient demonstrating mucinous neoplasia with peripheral DCIS foci at left. Similarly to morphological findings at mammography and ultrasonography, MMCs, at magnetic resonance imaging, are visualized as ovoid or lobulated masses with predominantly regular contours. The signal intensity is variable on T1-weighted images and with strong signal (similar to that of water or vessels) on T2-weighted images (Figure 3). Such high signal of the MMCs on T2-weighted images shows direct correlation with the degree of extracellular mucin and has high diagnostic sensitivity, although not pathognomonic, since it is also found in other lesions (secondary to necrosis, hemorrhage, edema, myxoid matrix or cystic component)(19). In addittion, the presence of intermediate signal on T2-weighted images suggests the mixed type of MMC. 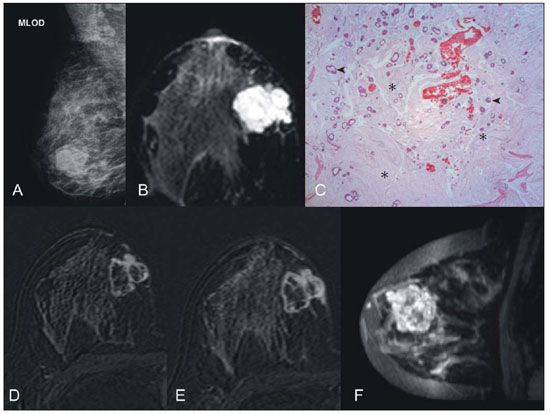 Figure 3. Female, 32-year-old patient with pure MMC. A: Mediolateral, oblique mammographic view demonstrating microlobulated ovoid nodule at the junction of the medial quadrants of the right breast. B: Axial MRI STIR image of the described nodule, demonstrating intense hypersignal onT2-weighted sequence with hypointense septa, common findings in mucinous carcinomas. C: Histological section at microscopy identifying mucinous tumor with a remarkable amount of mucin and little cellularity. D,E: Dynamic contrast-enhanced, T1-weighted images with fat suppression and subtraction, at the second and fifth minutes, demonstrating predominantly ring-shaped and progressive enhancement, and also marked enhancement of the septa within the lesion. F: Sagittal 3D reconstruction. On dynamic contrast-enhanced sequences, any enhancement morphology may occur, however, peripheral, ring-shaped or heterogeneous enhancement are more characteristic, and progressive along time (Figure 3). The pure type of MMC generally presents mild to moderate enhancement at the early phases, with centripetal tendency determining type 1 (progressive) curves, a feature demonstrated on Figure 4, or type 2 curve (plateau)(19). The progressive enhancement pattern is related to the tumor cellularity, nuclear grading, and amount of extracellular mucin. Thus, an intense enhancement in the first two minutes after gadolinium injection, or a type 3 curve (washout) must raise suspicion of mixed type MMC or, an even rarer pure tumor with high cellularity(10). 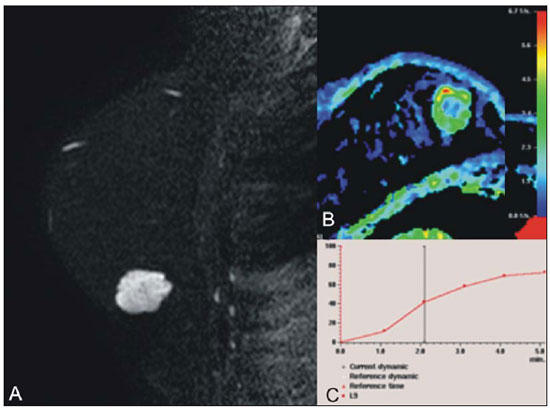 Figure 4. Female, 54-year-old patient with anatomopathological diagnosis of pure MMC. A: Sagittal T2-weighted SPAIR image demonstrating lobulated lesion with high signal on T2-weighted sequence. Color mapping of wash-in (B) and kinetic curve (C) showing slight enhancement at the early phase with progressive enhancement pattern. As compared with other subtypes of breast cancer, MMCs, in general, present low signal intensity on diffusion-weighted images. The quantitative analysis with measurement of the apparent diffusion coefficient (ADC) demonstrates high ADC values in relation to NS-IDCs. High ADC values may be associated with the presence of extracellular mucin and low tumor cellularity. However, such characteristic seems to be related to a larger extent to pure MMCs, which show high ADC value, similar or greater than benign lesions. Mixed mucinous tumors may exhibit lower ADC values, similarly to other breast cancer subtypes. Diffusion-weighted sequences and ADC calculation represent additional tools to increase the specificity of magnetic resonance imaging(19–21) (Figure 5). 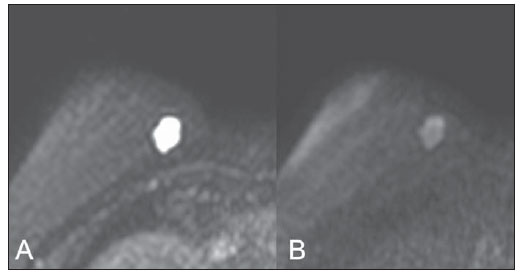 Figure 5. Female, 54-year-old patient with pure mucinous tumor represented on diffusion-weighted images, with b value = 0 on A and b = 700 on B, revealing minimum restriction, corroborated by the high signal intensity on C (ADC map). CONCLUSION Lesions suspicious for mucinous neoplasia prompt a careful evaluation of imaging findings, as the good correlation with histopathology has important implication in patient's prognosis. The imaging findings may suggest both subtypes of MMCs, pure and mixed ones. Mammography and ultrasonography allow a satisfactory evaluation of the lesion's morphology, but magnetic resonance imaging, besides providing data on morphology, also provides clues about tissue composition and along with the enhancement pattern after administration of gadolinium-based contrast medium, contribute to a better characterization of the lesion and to improve diagnostic accuracy. REFERENCES 1. Urban LABD, Schaefer MB, Duarte DL, et al. Recomendações do Colégio Brasileiro de Radiologia e Diagnóstico por Imagem, da Sociedade Brasileira de Mastologia e da Federação Brasileira das Associações de Ginecologia e Obstetrícia para rastreamento do câncer de mama por métodos de imagem. Radiol Bras. 2012;45:334–9. 2. Azevedo AC, Canella EO, Djahjah MCR, et al. Conduta das funcionárias de um hospital na adesão ao programa de prevenção do câncer de mama. Radiol Bras. 2012;45:215–8. 3. Moreira BL, Lima ENP, Bitencourt AGV, et al. Metástase na mama originada de carcinoma ovariano: relato de caso e revisão da literatura. Radiol Bras. 2012;45:123–5. 4. Vieira SC, Silva JS, Madeira EB, et al. Hemangioma de mama simulando metástase no PET-CT. Radiol Bras. 2011;44:401–2. 5. Yoo JL, Woo OH, Kim YK, et al. Can MR imaging contribute in characterizing well-circumscribed breast carcinomas? Radiographics. 2010;30:1689–702. 6. Rosen PP. Rosen's breast pathology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2009. 7. Bae SY, Choi MY, Cho DH, et al. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011;14:308–13. 8. Bode MK, Rissanen T. Imaging findings and accuracy of core needle biopsy in mucinous carcinoma of the breast. Acta Radiol. 2011;52:128–33. 9. Gobbi H. Carcinoma mucinoso invasor da mama e seus diagnósticos diferenciais na biópsia percutânea com agulha grossa [Editorial]. J Bras Patol Med Lab. 2010;46(2). 10. Monzawa S, Yokokawa M, Sakuma T, et al. Mucinous carcinoma of the breast: MRI features of pure and mixed forms with histopathologic correlation. AJR Am J Roentgenol. 2009;192:W125–31. 11. Wilson TE, Helvie MA, Oberman HA, et al. Pure and mixed mucinous carcinoma of the breast: pathologic basis for differences in mammographic appearance. AJR Am J Roentgenol. 1995;165:285–9. 12. Cao AY, He M, Liu ZB, et al. Outcome of pure mucinous breast carcinoma compared to infiltrating ductal carcinoma: a population-based study from China. Ann Surg Oncol. 2012;19:3019–27. 13. Vianna AD, Gasparetto TD, Torres GC, et al. Cancerização de lóbulos: correlação de achados mamográficos e histológicos. Radiol Bras. 2011;44:275–8. 14. Oliveira FGFT, Fonseca LMB, Koch HA. Responsabilidade civil do radiologista no diagnóstico do câncer de mama através do exame de mamografia. Radiol Bras. 2011;44:183–7. 15. Miranda CMNR, Santos CJJ, Maranhão CPM, et al. A tomografia computadorizada multislice é ferramenta importante para o estadiamento e seguimento do câncer de mama? Radiol Bras. 2012;45:105–12. 16. Conant EF, Dillon RL, Palazzo J, et al. Imaging findings in mucin-containing carcinomas of the breast: correlation with pathologic features. AJR Am J Roentgenol. 1994;163:821–4. 17. Lam WW, Chu WC, Tse GM, et al. Sonographic appearance of mucinous carcinoma of the breast. AJR Am J Roentgenol. 2004;182:1069–74. 18. Calas MJG, Alvarenga AV, Gutfilen B, et al. Avaliação de parâmetros morfométricos calculados a partir do contorno de lesões de mama em ultrassonografias na distinção das categorias do sistema BI-RADS. Radiol Bras. 2011;44:289–96. 19. Woodhams R, Kakita S, Hata H, et al. Diffusion-weighted imaging of mucinous carcinoma of the breast: evaluation of apparent diffusion coefficient and signal intensity in correlation with histologic findings. AJR Am J Roentgenol. 2009;193:260–6. 20. Marques EF, Medeiros MLL, Souza JA, et al. Indicações de ressonância magnética das mamas em um centro de referência em oncologia. Radiol Bras. 2011;44:363–6. 21. Barra FR, Barra RR, Barra Sobrinho A. Novos métodos funcionais na avaliação de lesões mamárias. Radiol Bras. 2012;45:340–4. 1. Radiologists, MDs, Residents at Centro de Ciências das Imagens e Física Médica – Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HC-FMRPUSP), Ribeirão Preto, SP, Brazil 2. Pathologist, Physician Assistant, Department of Pathology, Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRPUSP), Ribeirão Preto, SP, Brazil 3. PhD, Professor, Department of Gynecology and Obstetrics, Mastology Division, Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRPUSP), Ribeirão Preto, SP, Brazil 4. MD, Radiologist, Centro de Ciências das Imagens e Física Médica – Hospital das Clínicas daFaculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HC-FMRPUSP), Ribeirão Preto, SP, Brazil Mailing Address: Dr. Gustavo Nunes Medina Coeli Avenida Anna Cardoso de Miranda, 185, Jardim Inconfidência Uberlândia, MG, Brazil, 38411-231 E-mail: gustavonmc@yahoo.com.br Received September 26, 2012. Accepted after revision March 7, 2013. * Study developed at Centro de Ciências das Imagens e Física Médica – Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRPUSP), Ribeirão Preto, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554