Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 45 nº 6 - Nov. / Dec. of 2012
Vol. 45 nº 6 - Nov. / Dec. of 2012
|
ORIGINAL ARTICLE
|
|
Value of PET/CT in the approach to head and neck cancer |
|
|
Autho(rs): Otávio Alberto Curioni1; Ricardo Pires de Souza2; Ali Amar3; Débora Viana4; Abrão Rapoport5; Rogério Aparecido Dedivitis6; Claudio Roberto Cernea7; Lenine Garcia Brandão8 |
|
|
Keywords: Emission computed tomography; Fluorodeoxyglucose 18F; Head and neck neoplasms; Neoplasm staging; Squamous cell carcinoma. |
|
|
Abstract: INTRODUCTION
More than 630,000 new cases and approximately 350,000 deaths caused by head and neck cancer are estimated to occur every year in the world. The standardized rates of incidence correspond to15.3 per 100,000 men and 4.5 per 100,000 women(1). The treatment of head and neck epidermoid carcinoma is variable according to the anatomic location of the tumor and stage of the disease. For most cases at early stages, surgical resection is the first choice treatment, while for advanced lesions combined treatment is required by associating radiotherapy and/or chemotherapy(2). In Brazil, the five-year survival after the diagnosis of mouth and oropharynx cancer still remains below 50%, reflecting results obtained in other centers(3,4). Innovations have recently been introduced to improve clinical outcomes in the treatment of head and neck cancer. New radiotherapy techniques, such as modulated intensity radiotherapy (MIRT), image-guided radiotherapy (IGRT), utilization of targeted chemotherapy agents(5) and new surgical techniques, including sentinel lymph node biopsy and microsurgical reconstruction techniques have presented potential for improving therapeutic outcomes. Additionally to such innovations, the implementation of imaging diagnosis methods such as, among others, positron emission tomography (PET) associated with computed tomography (CT) with image fusion (PET/CT) have suggested a positive impact on treatment results, for providing, probably, a better definition of the extent of both primary and metastatic diseases. Such method capabilities may eventually lead to a more complete staging of a de novo disease and to a better follow-up of such patients, particularly in the early identification of disease recurrence, which, on its turn, might eventually represent an important tool in the prognostic evaluation. Imaging with 18-fluorodeoxyglucose (18FDG), the most utilized radiopharmaceutical, although not the single one – in the performance of PET/CT has become the standard for many malignant diseases, particularly because of its high sensitivity and high negative predictive value, thus allowing changes in therapeutic decisions, particularly in comparison with conventional methods, such as PET alone (without CT fusion imaging), CT alone, and magnetic resonance imaging (MRI)(6). In spite of evidences found in some publications, the utilization of 18FDG-PET/CT in head and neck deserves some considerations. An example is the non inclusion of such imaging method in the protocols of the National Comprehensive Cancer Network as part of routine staging. Although PET/CT is not globally accepted in staging protocols and cooperative groups, a reasonable number of publications approach the role of this imaging method in the management of head and neck cancer. In head and neck, several situations are presented as potential conditions where PET/CT might theoretically be useful. In head and neck oncology, the main clinical situations comprise the initial staging and investigation of lymph node metastatic disease(7,8) and hematogenic disease, detection of primary lesions in cases of occult primary tumors(9), evaluation of response to radiotherapy and/or chemotherapy, and investigation of early recurrence of disease and second primary tumor. In all such conditions changes in the approach may potentially occur(10). In some series, the second tumor incidence ranged between 11.1% and 12.9%(11,12), while lymph node disease presented specificity of 87% to 100% and sensitivity between 47% and 100%(13). As from October 2009, the Department of Head and Neck Surgery and Otorhynolaryngology of Hospital Heliópolis started utilizing PET/CT for selected cases (either for initial staging, evaluation of post-treatment response, or investigation for recurrences or distant metastasis), whose usual evaluation methods were not safe with respect to prognosis of the neoplasms. Thus, the authors have developed a preliminary retrospective analysis of their experience with this imaging diagnosis method, in an attempt to evaluate its possible advantages. MATERIALS AND METHODS Retrospective analysis of PET/CT images and medical records of patients admitted to the Department of Head and Neck Surgery and Otorhynolaryngology of Hospital Heliópolis in the period from October 2009 to January 2012. Considering the retrospective nature of the present study, the patients' informed consent was not required. The present study sample comprised 41 men (65%) and 22 women (35%). The median age of the population was 56 years (ranging between 11 and 85 years). Among those patients, 46 presented head and neck epidermoid carcinoma, 11 (17%), thyroid cancer (7 cases of papillary carcinoma and 4 cases of medullary carcinoma) and 6 patients (10%), other histopathological diagnoses as follows: lymphoma (2), mucoepidermoid carcinoma (2), undifferentiated sarcoma (1) and metastatic breast ductal carcinoma (1). As regards primary tumor site, the upper aerodigestive tract predominated, with 39 cases (61%), followed by 11 cases in the thyroid gland (17%), cervical lymph node in 10 cases (16%) and three cases involving salivary glands (5%). The images interpretation is based on 18FDG uptake indices (standardized uptake value – SUV), which defines the quotient between 18FDG uptake in the lesion, and the mean uptake in the rest of the body. Such calculation is influenced by several factors, namely, delivered dose, patient weight, size and site of the lesion, blood glucose level, time elapsed between contrast administration and images acquisition, etc. The utilization of the index facilitates evolutive comparisons. It is useful to evaluate therapeutic response, clinical staging, investigation of recurrences or distant metastasis, as well as second primary tumor. Such index can also be used in the differentiation between benign and malignant tumors, with a cutoff value around 2.5–3.0. In the present study, clinical indications for PET/CT included pretreatment clinical staging in 14 cases (22%), evaluation of response to chemotherapy and/or radiotherapy in 22 cases (35%), evaluation of postoperative recurrence in 15 cases (24%), investigation for distant metastasis in 10 cases (16%), investigation for second primary tumor after delayed treatment in 2 cases (3%), as shown on Table 1. 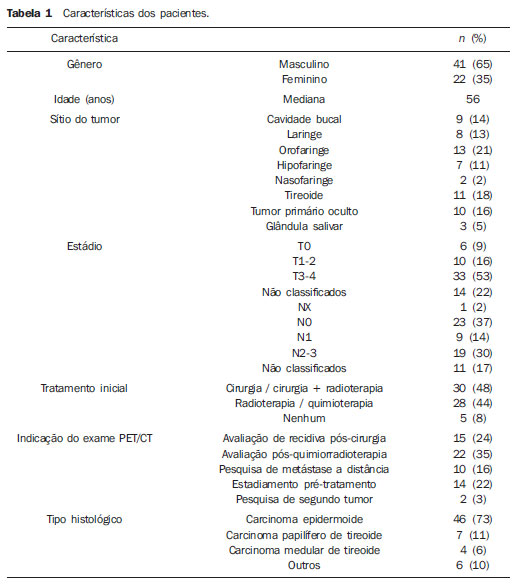 Hospital Heliópolis, by means of the São Paulo State Secretary of Health, has discretionary access to PET/CT for its patients in the Sistema Único de Saúde – SUS (Brazilian Unified Health System) network. In most of the 63 evaluated cases (n = 61), whole body CT images were acquired approximately one hour after intravenous administration of 18F-FDG, by means of an 8-detector-row CT apparatus, without intravenous administration of iodinated contrast medium. Subsequently, new whole body images were acquired by means of a PET tomograph with BGO detector in 3D mode. Finally, tomographic reconstruction and fusion of images obtained from the two modalities were performed, being duly processed and distributed along the axial, coronal and sagittal axes (Figure 1). 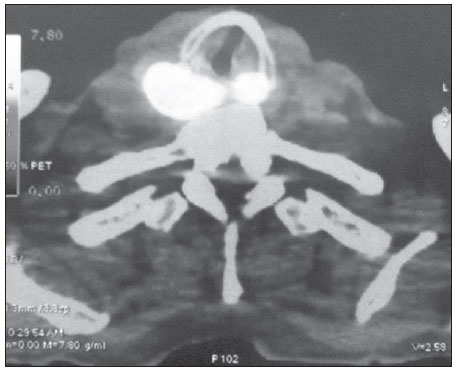 Figure 1. PET/CT. Expansible/infiltrative mass with intense uptake (FDG SUV max = 15.4) in the posterior hypopharynx wall and retropharyngeal space. RESULTS Amongst the 63 patients, 15 (24%) presented negative PET/CT results. Of those patients, 11 had been submitted to surgical treatment and 4 cases to chemotherapy and/or radiotherapy. In the group submitted to surgical intervention, PET/CT ruled out postoperative recurrence (4 cases), second tumor or distant metastasis (4 cases), and pre-treatment clinical staging (3 cases). In the remaining cases, PET/CT was indicated for analysis of post-chemoradiotherapy response (4 cases). None of those patients presented changes in the treatment plan after the negative study, and continued under regular clinical follow-up with no disease at the time of the present study's completion. Seven cases (11%) were considered as being false-positive – five post-chemoradiotherapy cases and two postoperative cases – all with SUV < 5.0. At the time of the present review completion, the patients were under follow-up, with no sign of disease. DISCUSSION More than 70% of patients with head and neck cancer present with advanced disease (clinical stage 3–4). In such a circumstance the determination of the locoregional extent of the disease as well as the identification of distant metastasis is of paramount importance. The correct staging will be useful in the decision making process, avoiding possible unnecessary interventions. Recent studies have attempted to demonstrate the impact of PET/CT on the staging, therapeutic decision making and on the analysis of patient survival. Lonneux et al.(10) have developed a prospective study aimed at evaluating the use of PET/CT in the initial staging of head and neck cancer and its impact on the therapeutic decision making. The authors have found disagreement in relation to initial staging in 43% of the cases. The use of PET/CT resulted in change to a more advanced stage of disease in 30% of the cases, and in change to a less advanced stage in 13%. Additionally, the study results caused changes in therapeutic planning for 13.7% of the patients. In a retrospective study Wong et al. have demonstrated that PET/CT has a good accuracy and good predictive value in the determination of lymph nodes condition. Additionally, the measurement of the tumor mass SUV can be an indicator of global survival(11). In the present series, PET/CT was utilized in 14 cases (22%) for pre-treatment clinical staging when the method was included in the protocols. In 3 cases (21%) there were changes in the clinical stage as a result of the diagnosis of two cases with pulmonary metastasis, and one case where a previously occult tumor was detected. Another challenging clinical situation is the assessment of therapeutic response, when there is still little clinical information available. In small series, PET/CT seems to offer advantages in the detection of recurrent or persistent disease, with specificity and sensitivity close to 100%, as compared with 75% for CT or MRI(11,12). In the present study, PET/CT was utilized for assessment of response to radiotherapy (either in association or not with chemotherapy) in 22 cases (35%). In 4/22 (18%) cases the result was negative and the patients are current under follow-up on an outpatient basis. The remaining 18 cases show positive results, with 5/22 (23%) false-positive cases (SUV < 5.0), 3 of them histologically confirmed and 2 cases clinically confirmed. Nine cases (41%) are currently under palliative care, and four cases (18%) were submitted to surgical rescue and are presently under follow-up on an out-patient basis. In 15 cases where routine exams did not define recurrence, PET/CT was utilized. Four cases (4/15 = 27%) were negative and the patients are under followed-up without the disease. The remaining studies (11/15 = 73%) were positive, with 6 cases being under palliative care and 5 having been rescued. Ten studies were performed for assessment of distant metastasis, with three cases of epidermoid carcinoma and seven cases of thyroid carcinoma (four medullary tumors and three papillary tumors). In 3/10 (30%) cases the study was negative and in 7/10 (70%) cases the study detected distant metastasis. In two cases (3%), the studies were requested to investigate a second primary tumor in patients treated for head and neck epidermoid carcinoma more than 36 months before, who presented wasting syndrome. In one case, a second primary tumor was identified in the lung, and the other was negative. Andrade et al.(13) have studied 28 cases of advanced post-chemoradiotherapy head and neck cancer by means of PET/CT and found sensitivity and specificity of 77% and 93%, respectively, with median followup time of 17.6 months. Three cases were false-negative and one, false-positive in studies performed eight weeks after treatment completion. PET/CT can provide data on disease extent, therapeutic targets and can identify early recurrence in head and neck cancer patients. However, large series of cases are necessary to clearly define frequency and interval protocols for evaluations with PET/CT. The establishment of an ideal timing for post-treatment performance of PET/CT would minimize the errors associated with physiological uptake related to the inflammatory effect versus persistence of the disease. Finally, some questions must be approached before PET/CT can be clearly allocated in the range of diagnostic protocols. The first question refers to the utilization of different radiopharmaceuticals (18F-fluorodeoxy-2-glucose is most frequently utilized, however several other radiomarkers have been utilized or tested), which would actually configure different exams, each one with its own effectiveness, thus generating a high complexity in the evaluation of results. The second question refers to the high cost and need for a highly complex physical structure for the production of radiopharmaceuticals and performance of the scans, which immediately stratifies the utilization of the method, restricting it to large centers. And finally, but not less important, the still disparate and often inconsistent results found in the literature about different clinical situations, either because of small-sized series or results which in fact, do not significantly change those already obtained by conventional methods, representing a factor that significantly and negatively affect the evaluation of the role of PET/CT in protocols for approaching malignant head and neck diseases. Even though the future looks promising, resources and efforts will be necessary to achieve a comfort level with respect to the role of PET/CT. CONCLUSION PET/CT demonstrates to be a potentially valuable method in the assessment of head and neck cancer patients. However, further studies with a higher number of cases will be necessary to allow the definition of a protocol of utilization. REFERENCES 1. Globocan 2008. Fast stats. Most frequent cancers: both sexes. [cited 2012 July 23]. Available from: http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900#BOTH 2. Forastiere A, Koch W, Tortti A, et al. Head and neck cancer. N Engl J Med. 2001;345:1890–900. 3. Carvalho AL, Ikeda MK, Magrin J, et al. Trends of oral and oropharyngeal cancer survival over five decades in 3267 patients treated in a single institution. Oral Oncol. 2004;40:71–6. 4. Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007;4:156–71. 5. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. 6. Poeppel TD, Krause BJ, Heusner TA, et al. PET/CT for the staging and follow-up of patients with malignancies. Eur J Radiol. 2009;70:382–92. 7. Kim SY, Roh JL, Yeo NK, et al. Combined 18F-fluorodeoxyglucose-positron emission tomography and computed tomography as a primary screening method for detecting second primary cancers and distant metastasis in patients with head and neck cancer. Ann Oncol. 2007;18:1698–703. 8. Yen TC, Chang JT, Ng SH, et al. The value of 18FFDG PET in the detection of stage M0 carcinoma of the nasopharynx. J Nucl Med. 2005;46:405–10. 9. Zanation AM, Sutton DK, Couch ME, et al. Use, accuracy, and implications for patient management of [18F]-2-fluorodeoxyglucose-positron emission/computerized tomography for head and neck tumors. Laryngoscope. 2005;115:1186–90. 10. Lonneux M, Hamoir M, Reychler H, et al. Positron emission tomography with [18F]fluorodeoxyglucose improves staging and management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol. 2010;28:1190–5. 11. Wong RJ, Lin DT, Schöder H, et al. Diagnostic and prognostic value of [(18)F]fluorodeoxyglucose positron emission tomography for recurrent head and neck squamous cell carcinoma. J Clin Oncol. 2002;20:4199–208. 12. Lowe VJ, Boyd JH, Dunphy FR, et al. Surveillance for recurrent head and neck cancer using positron emission tomography. J Clin Oncol. 2000;18:651–8. 13. Andrade RS, Heron DE, Degirmenci B, et al. Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys. 2006;65:1315–22. 1. PhD, Chief of the Service of Head and Neck Surgery and Otorhynolaryngology, Hospital Heliópolis, São Paulo, SP, Brazil. 2. PhD, Assistant, Service of Radiology, Hospital Heliópolis, São Paulo, SP, Brazil. 3. PhD, Surgeon, Department of Head and Neck Surgery and Otorhynolaryngology, Hospital Heliópolis, São Paulo, SP, Brazil. 4. MD, Resident, Service of Head and Neck Surgery and Otorhynolaryngology, Hospital Heliópolis, São Paulo, SP, Brazil. 5. Private Docent, Professor, Technical Director, Hospital Heliópolis, São Paulo, SP, Brazil. 6. Private Docent, Supervisor for the Group of Larynx and Hypopharynx, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil. 7. Associate Professor, Department of Head and Neck Surgery, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil. 8. Full Professor, Department of Head and Neck Surgery, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil. Mailing Address: Dr. Rogério A. Dedivitis Rua Cônego Xavier, 276, Cidade Nova Heliópolis São Paulo, SP, Brazil, 04231-030 E-mail: dedivitis.hns@uol.com.br / dedivitis@usp.br Received June 15, 2012. Accepted after revision September 11, 2012. * Study developed in the Service of Head and Neck Surgery and Otorhynolaryngology, and Service of Radiology at Hospital Heliópolis, and in the Department of Head and Neck Surgery at Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554