INTRODUCTION
In some regions of the world, congenital malformations represent the first cause of neonatal deaths(1). Approximately 20% of gestations with malformed fetuses progress to spontaneous miscarriage, and the remaining 80% will result in stillbirths or live births and, out of the latter, 3% to 5% will result in neonates with congenital anomalies(2,3). In Brazil, such malformations represent the second cause of infant mortality, determining 11.2% of such deaths(4). Congenital central nervous system (CNS) malformations are highly prevalent, affecting 1 to 10:1,000 live newborns(5). Such statistics may vary seasonally among countries and ethnic groups or among services of prenatal diagnosis and neonatology.
Approximately 21% of congenital malformations involve the CNS, constituting one of the most common congenital defects and may occur either isolatedly or in association with other malformations of the CNS itself or of other organs or system(6).
Currently, most of congenital anomalies can be diagnosed by means of obstetric ultrasonography and the fetal medicine seeks to establish an intrauterine fetal therapy for some episodes. Considering that an early diagnosis has significant repercussions on the neonatal prognosis, the present study has proposed to identify the prevalence of CNS malformations and associated malformations diagnosed by obstetric ultrasonography.
MATERIALS AND METHODS
Observational, cross-sectional, descriptive study developed in a public institution of reference for high-risk gestations. The present article is part of a more comprehensive project –
Estudo Colaborativo Latino-Americano de Malformações Congênitas (ECLAMC) (Latin American Collaborative Study of Congenital Malformations) –, a case-control study aimed at investigating epidemiological, clinical and imaging variables of malformed neonates. In Brazil, such network operates in 32 hospitals(7), and one of them is the institution where the present study was developed. The study project was approved by the Committee for Ethics in Research of the institution, respecting all the international principles on research involving humans. The present study was based on data from the ECLAMC. Conflicting interests: none declared.
The present study considered all the records of both stillborns and live newborns with congenital CNS malformations, either with diagnosis notified on medical records or live birth statements over a sixteen-month period. Neonates born in other hospitals, who for any reason were later assisted in the studied institution were excluded. The cases of congenital CNS malformations were identified according to their clinical and imaging presentations, being classified as isolated malformations; syndrome components; or malformations associated with other CNS anomalies or anomalies in other organs and systems.
The postnatal diagnosis was performed by means of physical examination, postnatal ultrasonography and/or computed tomography which, in association, were considered as the "gold standard" for a definitive diagnosis of congenital malformation, which is defined as an anatomical, physical defect diagnosed at birth and listed on the "Chapter XVII: Congenital malformations, deformations and chromosomal anomalies (Q00–Q99)" of the International Classification of Diseases – 10th revision. Thus, with a definitive diagnosis of malformation documented on medical records, and utilizing the described gold standard, the authors have retrospectively sough to confirm or reject the results of the obstetric imaging study, and then verify whether the method could or not detect malformations in neonates, determining the sensitivity and rate of false-negative results of obstetric ultrasonography in the study of fetal CNS malformations.
The SPSS (Statistical Package for Social Sciences) testing version 18.0 was utilized for data tabulation and statistical analysis. The categorical variables were described as simple frequencies and percentages. For the quantitative variables, means and standard deviations were utilized. The ultrasonography scans were performed by different professionals with more than 5-year experience in the method and with different apparatuses as follows: Voluson 730 Pro 3D/4D, Logic T5 GE and SonoSite MicroMaxx portable ultrasonography Machine. Two-dimensional evaluation, volumetric evaluation and ultrasound Doppler were utilized.
RESULTS
In the study period, 126 cases of congenital malformations were evaluated, and the frequency of congenital CNS malformations corresponded to 31.8% (40 cases). The same percentage was found for orthopedic malformations, followed by craniofacial malformations (20%) and other malformations (16%).
On average, the birth-weight of the 40 neonates with congenital CNS malformations was 2694.6 ± 872.8 g, ranging between 1000 and 4760 g. The frequency of male newborns was 55.3%, female newborns, 39.5%, and intersexed newborns, 5.3%. Only one case of stillborn was observed among the malformed neonates. The mean age of the puerperas was 27.2 ± 7.7 years, ranging between 13 and 43, and the mean age of the fathers was 31.6 ± 8.7 years, ranging between 18 and 55 years.
Congenital CNS malformations were isolated in most of cases, while in 37.5% (15 cases) there was association with other congenital CNS malformations. Hydrocephalus was the most prevalent malformation, followed by myelomeningocele; corpus callosum agenesis; anencephaly; and encephalocele. Dandy-Walker syndrome and holoprosencephaly presented a prevalence of 7.5% each. As regards the other malformations, a lower absolute frequency was observed, as shown on Table 1. Figure 1 shows cerebellar vermis agenesis with separation of the hemispheres and enlarged cisterna magna communicating with the fourth ventricle (findings of Dandy-Walker syndrome).
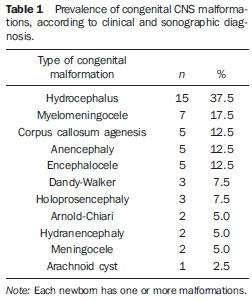
Figure 1. Cerebellar vermis agenesis with separation of the hemispheres and enlarged cisterna magna communicating with the fourth ventricle (findings of Dandy-Walker syndrome). Twodimensional (A) and three-dimensional (B) images.
Among the cases of anencephaly, 80% were observed in female newborns. Familial history of malformed newborns was found in 60% of cases of anencephaly and in 23.1% of all the cases with such antecedent.
As regards the presence of congenital malformations in other organs or systems, 57.5% of the newborns presented one or more congenital defects in association with CNS malformation. Most prevalent sites of congenital malformations were the following: craniofacial, followed by orthopedic, cardiovascular, genitourinary and gastrointestinal malformations (Table 2). Patau syndrome occurred in one case of holoprosencephaly. Figure 2 shows proboscis, an anomaly that is also characteristic of trisomy 13, which almost always is associated with holoprosencephaly, together with hypotelorism, bilateral or median cleftlip and palate, single nostril, cyclopia or even microphthalmos.
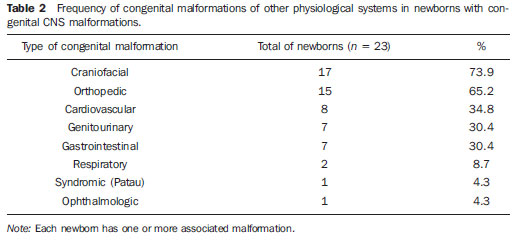
Figure 2. Proboscis, an anomaly that is also characteristic of trisomy 13, which almost always is associated with holoprosencephaly. Two-dimensional (A) and three-dimensional (B) images.
Hydrocephalus was the congenital malformation most frequently associated with either myelomeningocele, or corpus callosum agenesis or encephalocele (Figure 3).
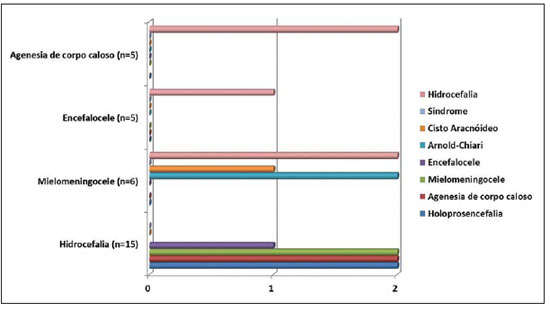
Figure 3. Distribution of other congenital CNS malformations associated with hydrocephalus, myielomeningocele, encephalocele and corpus callosum agenesis.
As regards other congenital malformations associated to CNS malformations, craniofacial, followed by orthopedic and cardiovascular malformations were most frequently found (Figure 4).
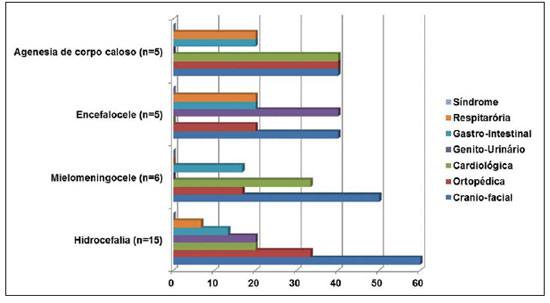
Figure 4. Distribution of congenital defects of other systems in association with more prevalent CNS malformations.
The sonographic sensitivity in the investigation of fetal CNS malformations was of 79.4%. The rate of false-negative results reached 20.5%. Among quantifiable limitations, oligohydramnios is highlighted, being present in 25% of false-negative sonographic results.
DISCUSSION
The frequency of congenital CNS malformations among all the evaluated cases of malformations was 31.8%, a rate similar to the ones reported by some authors(8,9) and higher than the one found by Noronha et al.(5) and by Pitkin(6), 13% and 21% of cases, respectively. Differently from the findings reported by Pitkin(6) and by Victora and Barros(4), congenital CNS malformations were the most frequent ones, together with orthopedic malformations, followed by craniofacial malformations. Another study reports craniofacial and limbs malformations as most frequently found(10). A case-control study indicates that the etiopathogenesis of some congenital orthopedic malformations may involve neurological factors producing alterations in the spinal cord or in nerves(11).
Although some studies report cardiovascular anomalies as most common malformations, marked differences in study populations, as well as in criteria and diagnostic method utilized may lead to under-diagnosis of mild defects. Thus, a higher prevalence of such congenital malformations has been observed as routine echocardiography is utilized – an unusual practice in most health services(12).
Among congenital CNS malformations, hydrocephalus was the most frequent, followed by myelomeningocele, as reported by a retrospective study(13). In sequence, anencephaly together with corpus callosum agenesis and encephalocele were found to be more frequent, in disagreement with the frequencies described by Moore & Persaud(14). Among neural tube defects, myelomeningocele, anencephaly and encephalocele were observed in descent order. Certain authors report the same tendency(9,15).
In cases of anencephaly, female newborns were affected at a 4:1 ratio that was superior to the ratio observed in some studies(16,17). Three of five cases presented familial history of malformations, a number that was higher than the one reported by Ramos et al.(18).
Dávila-Gutiérrez reports a relation between hydrocephalus and corpus callosum agenesis(19). On the other hand, other authors report cases of concomitance of hydrocephalus and myelomeningocele(20,21), while Levey et al. report coexistence of hydrocephalus and holoprosencephaly(22). In the present study, such associations were observed with higher frequency.
Congenital craniofacial malformations were more prevalent in cases of hydrocephalus, as corroborated by Cinalli et al.(23), and myelomeningocele. Congenital cardiologic malformations were most frequently present in cases of corpus callosum agenesis, in agreement with the findings of Mowat et al.(24). On the other hand, genitourinary malformations were most frequently found in cases of encephalocele, in agreement with Rittler et al.(25). Patau syndrome – a very rare chromosomal abnormality, with an incidence of 1/5000 to 1/20000 births – occurred in a case of holoprosencephaly. Such association is described in the literature(26).
The sonographic sensitivity in the investigation of fetal CNS malformations was of 79.4%. Randomized studies estimate a sonographic sensitivity of about 80% for detecting CNS malformations in cases of high-risk gestation(27,28). It is known that factors such as quality of apparatuses, sound waves interaction with tissues, examination techniques – appropriate adjustment of gain waveforms, for example –, dedicated scan time, experience and knowledge of the medical sonographer, besides factors such as high maternal body mass índex, fetal statics, advanced gestational age and decreased amniotic fluid index, may change the method sensitivity. Constant perfecting and deep knowledge of specialized methods such as Doppler(29,30) and volumetric (3D/4D) ultrasonography, in association with the increasing technological development with state-of-the-art equipment, have contributed to a good sensitivity of the method in the screening for malformations, with prognostic repercussions.
The rate of false-negative results was 20.5%. Among the quantifiable limitations, oligohydramnios is highlighted, being present in 25% of false-negative sonographic studies. Magnetic resonance imaging complementary to ultrasonography, differently from the latter, demonstrates improved diagnostic accuracy with the gestational age progress, and is not affected by decreased amniotic fluid levels, maternal obesity or fetal statics (31). Additionally, one of the main contributions of magnetic resonance imaging is to complement the role of ultrasonography in the study of fetal CNS malformations which is complicated at late phases of gestation because of the advanced cranial bones ossification. Even so, it should be stressed that the utilization of magnetic resonance imaging is restricted to complement ultrasonography, considering the limitations of the method such as high cost, fetal motion artifacts, claustrophobia, the recommendation not to perform the method in the first gestational trimester(32), besides its lesser availability.
Thus, ultrasonography provides early diagnosis with good sensitivity, which in association with the accessibility and availability of the method, has contributed to consolidate its role as the modality of choice in the routine screening for fetal CNS malformations. However, considering the inherent method limitations, continuity of improvements should still be encouraged in the search for excellence in early diagnosis, in order to highlight novel technologies to supplement the assessment of the uterine contents. In this context, a wide availability of fetal magnetic resonance imaging for the study population has been sought in order to allow additional diagnostic findings, as reported in the literature(32,33).
CONCLUSIONS
Obstetric ultrasonography demonstrates good sensitivity in the screening for fetal CNS malformations, especially with the constant improvements and increased knowledge of specialized methods such as Doppler and volumetric ultrasonography (3D/4D), contributing to consolidate its role as a modality of choice in this routine. As a complementary method, magnetic resonance imaging may be useful, providing information for an even better perinatal assistance.
REFERENCES
1. Rosano A, Botto LD, Botting B, et al. Infant mortality and congenital anomalies from 1950 to 1994: an international perspective. J Epidemiol Community Health. 2000;54:660–6.
2. Rankin J, Pattenden S, Abramsky L, et al. Prevalence of congenital anomalies in five British regions, 1991-99. Arch Dis Child Fetal Neonatal Ed. 2005;90:F374–9.
3. Nikkilä A, Rydhstroem H, Källén B, et al. Ultrasound screening for fetal anomalies in southern Sweden: a population-based study. Acta Obstet Gynecol Scand. 2006;85:688–93.
4. Victora CG, Barros FC. Infant mortality due to perinatal causes in Brazil: trends, regional patterns and possible interventions. São Paulo Med J. 2001;119:33–42.
5. Noronha L, Medeiros F, Martins VDM, et al. Malformações do sistema nervoso central: análise de 157 necrópsias pediátricas. Arq Neuropsiquiatr. 2000;58:890–6.
6. Pitkin RM. Folate and neural tube defects. Am J Clin Nutr. 2007;85:285S–288S.
7. Castilla EE, Orioli IM. ECLAMC: the Latin-American collaborative study of congenital malformations. Community Genet. 2004;7:76–94.
8. Moron AF. Diagnóstico pré-natal das malformações congênitas no contexto do sistema de saúde [tese]. São Paulo: Faculdade de Saúde Pública, Universidade São Paulo; 1995.
9. Costa CMS, Gama SGN, Leal MC. Congenital malformations in Rio de Janeiro, Brazil: prevalence and associated factors. Cad Saúde Pública. 2006;22:2423–31.
10. Castro MLS, Cunha CJ, Moreira PB, et al. Frequency of multiple neonatal malformations in Pelotas, Rio Grande do Sul, Brazil, and associated socio-demographic factors. Cad Saúde Pública. 2006;22:1009–15.
11. Guardiola A, Koltermann V, Aguiar PM, et al. Neurological congenital malformations in a tertiary hospital in south Brazil. Arq Neuropsiquiatr. 2009;67:807–11.
12. Goldmuntz E. The epidemiology and genetics of congenital heart disease. Clin Perinatol. 2001;28:1–10.
13. Pinar H, Tatevosyants N, Singer DB. Central nervous system malformations in a perinatal/neonatal autopsy series. Pediatr Dev Pathol. 1998;1:42–8.
14. Moore KL, Persaud TVN. Embriologia clínica. Rio de Janeiro, RJ: Guanabara Koogan; 2004.
15. Shurtleff DB, Lemire RJ. Epidemiology, etiologic factors, and prenatal diagnosis of open spinal dysraphism. Neurosurg Clin N Am. 1995;6:183–93.
16. Loncarek K, Mustac E, Frkovic A, et al. Prevalence of anencephaly in the region of Rijeka, Croatia. Eur J Epidemiol. 2001;17:241–4.
17. Verma M, Chhatwal J, Singh D. Congenital malformations – a retrospective study of 10,000 cases. Indian J Pediatr. 1991;58:245–52.
18. Ramos JLA, Laurindo VM, Vaz FAC, et al. Malformações congênitas: estudo prospectivo de dois anos em três maternidades de São Paulo. Pediat (S Paulo). 1981;3:20–8.
19. Dávila-Gutiérrez G. Agenesis and dysgenesis of the corpus callosum. Semin Pediatr Neurol. 2002;9:292–301.
20. Dicianno BE, Kurowski BG, Yang JM, et al. Rehabilitation and medical management of the adult with spina bifida. Am J Phys Med Rehabil. 2008;87:1027–50.
21. Shurtleff DB. 44 years' experience with management of myelomeningocele: presidential address, Society of Research into Hydrocephalus and Spina Bifida. Eur J Pediatr Surg. 2000;10 Suppl 1:5–8.
22. Levey EB, Stashinko E, Clegg NJ, et al. Management of children with holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:183–90.
23. Cinalli G, Sainte-Rose C, Kollar EM, et al. Hydrocephalus and craniosynostosis. J Neurosurg. 1998;88:209–14.
24. Mowat DR, Wilson MJ, Goossens M. Mowat-Wilson syndrome. J Med Genet. 2003;40:305–10.
25. Rittler M, López-Camelo JS, Castilla EE, et al. Preferential associations between oral clefts and other major congenital anomalies. Cleft Palate Craniofac J. 2008;45:525–32.
26. Lehman CD, Nyberg DA, Winter TC 3rd, et al. Trisomy 13 syndrome: prenatal US findings in a review of 33 cases. Radiology. 1995;194:217–22.
27. Crane JP, LeFevre ML, Winborn RC, et al. A randomized trial of prenatal ultrasonographic screening: impact on the detection, management, and outcome of anomalous fetuses. The RADIUS Study Group. Am J Obstet Gynecol. 1994;171:392–9.
28. Ewigman BG, Crane JP, Frigoletto FD, et al. Effect of prenatal ultrasound screening on perinatal outcome. RADIUS Study Group. N Engl J Med. 1993;329:821–7.
29. Gabriel ML, Piatto VB, Souza AS. Aplicação clínica da ultrassonografia craniana com Doppler em neonatos prematuros de muito baixo peso. Radiol Bras. 2010;43:213–8.
30. Moron AF, Milani HJF, Barreto EQS, et al. Análise da reprodutibilidade do Doppler de amplitude tridimensional na avaliação da circulação do cérebro fetal. Radiol Bras. 2010;43:369–74.
31. Prayer D, Brugger PC, Prayer L. Fetal MRI: techniques and protocols. Pediatr Radiol. 2004;34:685–93.
32. Ximenes RLS, Szejnfeld J, Ximenes ARS, et al. Avaliação crítica dos benefícios e limitações da ressonância magnética como método complementar no diagnóstico das malformações fetais. Radiol Bras. 2008;41:313–8.
33. Furtado AD, Pinto MVR, Rangel CC, et al. Confiabilidade da análise qualitativa da ressonância magnética do encéfalo em prematuros extremos. Radiol Bras. 2010;43:375–8.
1. MD, Physician, Department of Medicine, Universidade Federal de Sergipe (UFS), Aracaju, SE, Brazil.
2. MD, Resident in Radiology and Imaging Diagnosis, Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
3. Master, MD, Pediatrician, Assistant Professor, Department of Medicine, Universidade Federal de Sergipe (UFS), Aracaju, SE, Brazil.
4. MD, Fellow Master degree, Pediatrician, Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
5. Fetal Medicine Specialist, Preceptor of Residency in Radiology and Imaging Diagnosis, Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
6. MD, Radiologist, Coordinator for Medical Residency in Radiology and Imaging Diagnosis, Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
7. Fellow PhD degree, Titular Member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), Assistant Professor, Department of Medicine, Universidade Federal de Sergipe (UFS), Aracaju, SE, Brazil.
8. PhD, Neurosurgeon, Associate Professor, Department of Medicine, Universidade Federal de Sergipe (UFS), Aracaju, SE, Brazil.
Mailing Address:
Dr. Daniel Alvarenga Fernandes
Rua Buenos Aires, 726, Ed. América Central, ap. 1404, Jardim das Américas
Cuiabá, MT, Brazil, 78060-634
E-mail: daniel_alvafer@ yahoo.com.br
Received July 27, 2012.
Accepted after revision October 22, 2012.
* Study developed at Maternidade Nossa Senhora de Lourdes and Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
 Vol. 45 nº 6 - Nov. / Dec. of 2012
Vol. 45 nº 6 - Nov. / Dec. of 2012





