INTRODUCTION
Cholesteatoma is a benign cystic lesion of aggressive behavior, composed of keratinized stratified squamous epithelium. It is constituted of an epithelial matrix surrounded by an inflammatory stroma with variable thickness containing cellular debris(1). Cholesteatomas may be either congenital or acquired, according to their origin.
Congenital cholesteatomas frequently involve the cavity of the middle ear and the mastoid process, but may also affect other parts of the temporal bone, including the squama, petrous apex and the external auditory meatus. This entity originates at the moment of the neural tube closure as the ectoderma gets trapped in the temporal bone in an extradural situation. In cases where the ectoderma gets trapped in an intradural situation, the result will be the so called epidermoid inclusion cyst which has been described in different locations, the pontocerebellar angle being the most common one. By definition, in cases of congenital cholesteatoma, the tympanic membrane is intact and no sign of infection. In most of the cases, the lesion develops in the anterior mesotympanum or in the posterior eptympanum(1,2).
Acquired cholesteatomas, most frequently, originate from retraction of the posterosuperior quadrant (
pars flaccida) and, less commonly, of the lower portion (
pars tensa) of the tympanic membrane Acquired cholesteatomas may be divided into primary - related to tympanic membrane retraction, and secondary - related to epithelial migration towards the middle ear, in a site of tympanic membrane perforation, including iatrogenically during otological procedures.
Pars flaccid cholesteatomas progressively involve the Prussak's space and erodes the circumjacent structures such as the spur and the ossicular chain, principally the malleus head, the long process and the body of incus. After growth, the cholesteatoma invades the antrum and the mastoid process, eroding further structures of the middle ear such as the facial nerve canal, the
tegmen tympani and the posterior semicircular canal wall(1,2).
The etiopathogenesis of congenital and acquired cholesteatomas still remains under discussion, and there are several theories to explain the origins of such entity.
The treatment consists in surgical resection of the epithelial matrix. However, a high number of patients submitted to surgical treatment remain with residual or recurrent cholesteatoma, many times identified only at the second operative time. There may be a new growth of the cholesteatoma either from the non-resected epithelial matrix (residual cholesteatoma) or from the development of a new matrix on the retracted tympanic membrane resulting from scarring (recurrent cholesteatoma)(3).
The eradication of cholesteatomas has represented a challenge for surgeons. Several procedures have been utilized, either with open or closed surgical technique, but the ideal surgical method still remains controversial. The different techniques rely on the sparing or not of the posterior wall of the external auditory conduct, either connecting or not the mastoid cavity with the exterior.
Open surgical techniques include mastoidectomy (abrasion of the posterior wall of the external auditory meatus, with removal of remainders of tympanic membrane, malleus and incus, in association with meatoplasty); modified radical mastoidectomy (partial removal of the attic and of the posterior wall of the meatus); and radical mastoid cavity reconstruction (radical mastoidectomy with reconstruction of the tympanic bulla utilizing the temporal fascia). Among the closed surgical techniques, tympanotomy is aimed at creating an incision on the posterior wall of the external auditory meatus, in front of the facial nerve in order to remove the cholesteatoma near the stapes and the round window, while mastoidectomy with tympanoplasty is a procedure performed in a single surgical time in cases where there is no doubt on the total excision of the cholesteatoma. More conservative techniques present the disadvantage of requiring a revision surgery (second look), but leads to better outcome in relation to hearing preservation. According to several published studies, the incidence of residual or recurrent cholesteatomas seems to be lower with open surgical techniques(4,5).
IMAGING STUDIES
Computed tomography (CT) still remains as the method of choice for diagnosis and assessment of cholesteatomas extent. It can demonstrate ossicular erosion as well as possible complication such as erosion of tegmen tympani and lateral semicircular canal(6). Unfortunately, however, after surgery most of the patients present total or subtotal opacification of the middle ear, so it is not possible to identify the presence of any inflammatory process, abscess, scar or granulomatous tissue, cholesterol granuloma and cholesteatoma at CT(7).
As shown on Figures 1A to 1E, at CT, the patient with post-mastoidectomy residual cholesteatoma presents a mass with soft tissue density obstructing the surgical cavity which, at magnetic resonance imaging (MRI), demonstrated diffusion restriction at non echo-planar imaging. Peripheral enhancement is observed at delayed phase (45 minutes after contrast injection), spin echo, T1-weighted sequence with fat suppression. A second postoperative follow-up with these same sequences allows the demonstration, in a different moment, an intense contrast enhancement and absence of diffusion restriction compatible with the presence of surgically confirmed inflammatory granulation tissue.
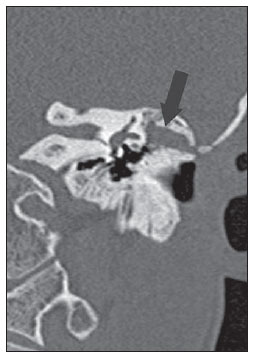
Figure 1A. CT, coronal plane: postoperative status of conservative mastoidectomy. Presence of material with soft tissue density obstructing the surgical cavity (arrow). The tomographic finding is nonspecific and might correspond either to fibrotic/inflammatory tissue or residual/recurrent cholesteatoma.
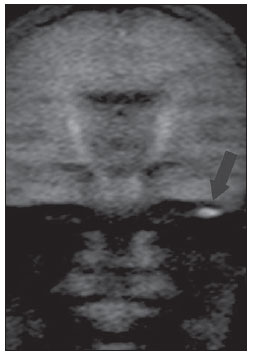
Figure 1B. MRI, coronal plane, HASTE diffusionweighted (non echo-planar) sequence. Note diffusion restriction in residual cholesteatoma at left (arrow).
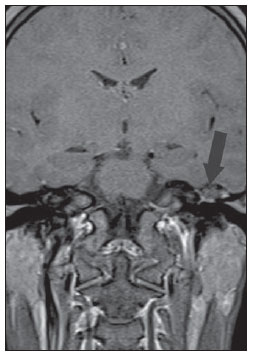
Figure 1C. MRI, coronal plane, spin echo, T1-weighted sequence with fat suppression, 45 after intravenous paramagnetic contrast injection. Notethe subtle peripheral contrast-enhancement, a typical finding of cholesteatoma (arrow).
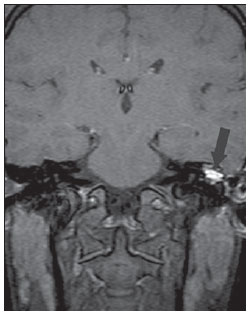
Figure 1D. The same patient, one year later. MRI, coronal plane, spin echo T1-weighted sequence with fat suppression, 45 minutes after intravenous paramagnetic contrast injection. Note the intense contrast-enhancement in the whole lesion, corresponding to inflammatory granulomatous tissue (arrow).
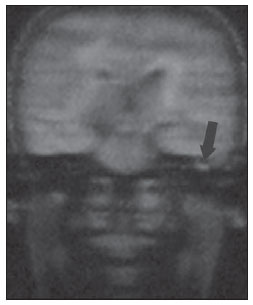
Figure 1E. The same patient, one year later. MRI, coronal plane, HASTE diffusion-weighted (non echo-planar) sequence. No diffusion restriction is observed in the inflammatory granulomatous tissue (arrow).
Recently, MRI including diffusion echo-planar imaging (EPI) has gained relevance in the diagnosis of cholesteatoma. Initially, such sequence was utilized in the assessment of cerebral ischemia. It is based on the demonstration of the movement of free water molecules and possible restriction to such a movement in pathological cases. Fitzek et al. were the first ones to demonstrate that cholesteatoma was visualized with high signal intensity at diffusion EPI sequences, more specifically at b1000 images. However, diffusion EPI presents low spatial resolution, relatively thick sections and higher susceptibility to artifacts in regions of air-bone interface, representing disadvantages which many times hinder the diagnosis of cholesteatomas, particularly in cases of lesions measuring < 5 mm in diameter(8). Such a limitation can be clearly observed on Figure 2D, where the artifact hinders the visualization of the focus of diffusion restriction, while at the image on Figure 2C acquired with non echo-planar sequence, the diffusion restriction was clearly demonstrated. Toyama et al., in a study with 17 patients, have found 91.5% sensitivity, 60% specificity, 84.6% positive predictive value, and 75% negative predictive value, concluding that diffusion EPI combined with contrast-enhanced imaging is useful in the differential diagnosis of recurrent cholesteatoma and granulation tissue(9).
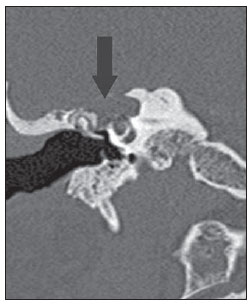
Figure 2A. CT, coronal plane. Congenital cholesteatoma involving the region of the right geniculate ganglion (arrow).
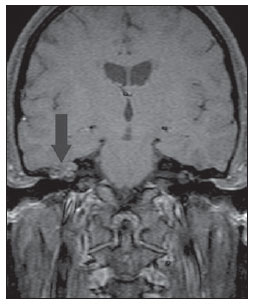
Figure 2B. MRI, coronal plane, spin echo, T1-weighted sequence with fat suppression, 45 minutes intravenous paramagnetic contrast injection. Note the subtle peripheral contrast-enhancement, a typical finding of cholesteatoma (arrow).
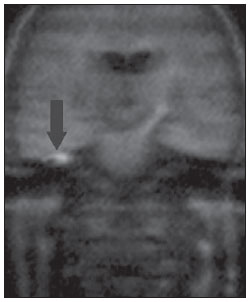
Figure 2C. MRI, coronal plane, HASTE diffusionweighted (non echo-planar) sequence. Note diffusion restriction in congenital cholesteatoma at right (arrow).
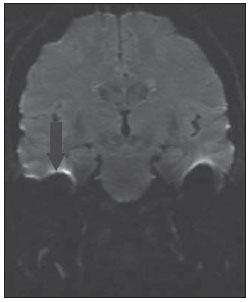
Figure 2D. MRI, coronal plane, echo-planar diffusion-weighted sequence. Note the presence of artifact on the interface with the floor of the middle cranial fossa, hindering the investigation of diffusion restriction in the ears (arrow).
Jeunen et al. have found sensitivity, specificity, PPV and NPV of 54%, 90%, 92% and 47%, respectively in a study involving 31 patients and utilizing diffusion EPI. In the case of residual or recurrent cholesteatomas, sensitivity, specificity, PPV and NPV were 83%, 82%, 83% and 82%, respectively. In such study, residual cholesteatomas were correctly identified in 15 out of 18 patients, and small-sized lesions (2-5 mm) were missed in three patients(10).
Recent studies have demonstrated the value of non-EPI diffusion weighted imaging in the diagnosis of primary cholesteatomas (Figures 2A to 2D) and also in postoperative recurrence. Single shot turbo spin echo (SSTSE) diffusion-weighted or multishot fast spin echo (FSE) imaging present lower susceptibility to artifacts and can be acquired with thinner sections, with higher spatial resolution (Figures 3A and 3B), allowing the detection of small-sized lesions, such as the one shown on Figure 4, with 3 mm in diameter. Dubrulle et al. have evaluated TSE diffusion-weighted imaging for detecting recurrent cholesteatomas, but the threshold for detection of small-sized cholesteatomas in this study was 5 mm, equal to the threshold for EPI sequences. Other studies indicate that SSTSE presents high sensitivity and specificity, detecting cholesteatomas with up to 2 mm in diameter(11). De Foer et al. have found 90% sensitivity, 100% specificity, 100% PPV, and 96% NPV for non-EPI sequences in the study of patients with residual cholesteatoma. In this study, cholesteatomas measuring 2 to 6 mm in diameter were diagnosed(6).
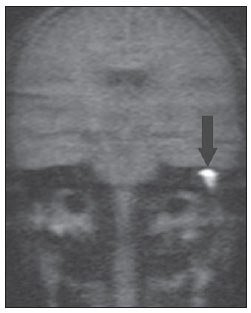
Figure 3A. MRI, coronal plane, HASTE (non echoplanar) sequence. Note diffusion restriction in cholesteatoma at right (arrow).
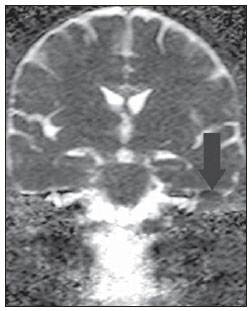
Figure 3B. ADC map of the same case confirming diffusion restriction (arrow).
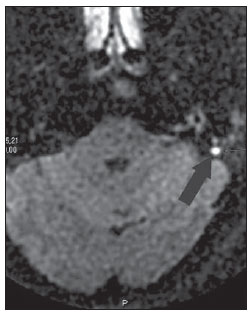
Figure 4. MRI, axial plane, PROPELER diffusionweighted (non echo-planar) sequence. Presence of a focus of diffusion restriction in a small (3 mm) cholesteatoma at left (arrow).
Non-EPI diffusion-weighted imaging acquisition time is longer than for echo-planar imaging and presents two significant limitations. Motion artifacts may blur hyperintense b1000 images, provoking signal iso-signal intensity and conditioning a false-negative result. Spontaneously evacuated cholesteatoma is also a possible cause o false-negative result, once the contents of desquamated and degraded keratin responsible for the diffusion restriction shall not be present in cases of automastoidectomy. In such cases, the cholesteatoma content is evacuated into the external auditory meatus, and can displace the matrix far away from its original positioning in the middle ear and in the mastoid antrum. Thus, it is important to highlight that both diffusion weighted EPI and non-EPI imaging may fail in the detection of cholesteatoma due to the absence of keratin (responsible for the hyper signal intensity).
Figures 5A and 5B exemplify such method limitation, demonstrating a case of bilateral acquired cholesteatoma with automastoidectomy at left. In such an example, non-echo-planar HASTE imaging demonstrated diffusion restriction at right and absence of restriction at left, despite the matrix permanence after auto evacuation of the keratin content.
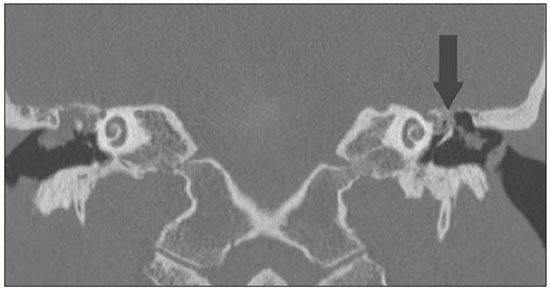
Figure 5A. CT, coronal plane. Bilateral acquired cholesteatoma with automastoidectomy at left (arrow).
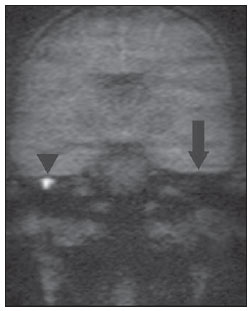
Figure 5B. MRI, coronal plane, HASTE diffusionweighted (non echo-planar) sequence. Note diffusion restriction in cholesteatoma at right (arrow head). There is no diffusion restriction at left, due to the method limitation to demonstrate the residual matrix in auto-evacuated cholesteatoma (arrow).
Images acquisition with MRI T1-weighted sequences with fat saturation, 30-45 minutes after gadolinium injection has been originally described by Williams and collaborators based on the fact that cholesteatoma is a non-enhancing avascular tissue whereas inflammatory, granulomatous and cicatricial tissues are poorly vascularized and do present slow contrast enhancement. Such T1-weighted sequence could demonstrate the exact location of the cholesteatoma in the middle ear and mastoid process, as well as the surrounding inflammatory reaction(2,4).
Ayache et al. have found 90% specificity and 100% sensitivity after utilizing contrast agent. However, residual pearls measuring up to 3 mm could not be diagnosed with such technique. The time required for images acquisition after contrast injection represents the main limitation of the technique, and it should be highlighted that early imaging may lead to false positive results for cholesteatoma(12).
The combination of post-contrast MRI with non-EPI diffusion-weighted sequences presents higher sensitivity and specificity than CT for detecting residual cholesteatomas, conditioning the reduction of the number of tympanic cavity revision surgeries. However, the utilization of non-EPI diffusion-weighted sequences without necessity of delayed contrast-enhanced imaging has been defended by some authors, since the combined utilization of such sequences adds little diagnostic sensitivity and specificity to the study. Also, diffusion-weighted imaging alone presents further advantages, namely, reduction of the total images acquisition time and costs reduction(13).
REFERENCES
1. Semaan MT, Megerian CA. The pathophysiology of cholesteatoma. Otolaryngol Clin North Am. 2006;39:1143-59.
2. Lemmerling M, De Foer B. Imaging of cholesteatomatous and non-cholesteatomatous middle eardisease. In: Lemmerling M, Kollias S, editors. Radiology of the petrous bone. Berlin: Springer; 2004. p. 31-47.
3. De Foer B, Vercruysse JP, Bernaerts A, et al. Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol. 2008;29:513-7.
4. Aquino JEAP, Cruz Filho NA, Aquino JNP. Cholesteatoma surgery in children and adolescents. Analysis in 200 patients. Intl Arch Otorhinolaryngol. 2006;10:55-61.
5. Dornelles C, Costa SS, Meurer L, et al. Some considerations about acquired adult and pediatric cholesteatomas. Rev Bras Otorrinolaringol. 2005;71:536-46.
6. De Foer B, Vercruysse JP, Bernaerts A, et al. The value of single-shot turbo spin-echo diffusionweighted MR imaging in the detection of middle ear cholesteatoma. Neuroradiology. 2007;49:841-8.
7. Lemmerling MM, De Foer B, Verbist BM, et al. Imaging of inflammatory and infectious diseases in the temporal bone. Neuroimaging Clin N Am. 2009;19:321-37.
8. Fitzek C, Mewes T, Fitzek S, et al. Diffusionweighted MRI of cholesteatomas of the petrous bone. J Magn Reson Imaging. 2002;15:636-41.
9. Toyama C, Leite CC, Baraúna Filho IS, et al. The role of magnetic resonance imaging in the postoperative management of cholesteatomas. Rev Bras Otorrinolaringol. 2008;74:693-6.
10. Jeunen G, Desloovere C, Hermans R, et al. The value of magnetic resonance imaging in the diagnosis of residual or recurrent acquired cholesteatoma after canal wall-up tympanoplasty. Otol Neurotol. 2008;29:16-8.
11. Dubrulle F, Souillard R, Chechin D, et al. Diffusion- weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology. 2006;238:604-10.
12. Ayache D, Williams MT, Lejeune D, et al. Usefulness of delayed postcontrast magnetic resonance imaging in the detection of residual cholesteatoma after canal wall-up tympanoplasty. Laryngoscope. 2005;115:607-10.
13. De Foer B, Vercruysse JP, Bernaerts A, et al. Middle ear cholesteatoma: non-echo-planar diffusion- weighted MR imaging versus delayed gadolinium-enhanced T1-weighted MR imaging - value in detection. Radiology. 2010;255: 866-72.
1. MD, Radiologist, Hospital Biocor, Belo Horizonte, MG, Brazil.
2. MD, Radiologist, Axial Medicina Diagnóstica, Belo Horizonte, MG, Brazil.
3. Titular member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), MD, Radiologist, Axial Medicina Diagnóstica, Belo Horizonte, MG, Brazil.
Mailing Address:
Dra. Marina Vimieiro Timponi de Moura
Rua Rio Grande do Norte, 501, Santa Efigênia
Belo Horizonte, MG, Brazil, 30130-130
E-mail: mvtimp@gmail.com
Received March 29, 2012.
Accepted after revision July 10, 2012.
* Study developed at Axial Medicina Diagnóstica, Belo Horizonte, MG, Brazil.
 Vol. 45 nº 5 - Sep. / Oct. of 2012
Vol. 45 nº 5 - Sep. / Oct. of 2012













