Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 45 nº 5 - Sep. / Oct. of 2012
Vol. 45 nº 5 - Sep. / Oct. of 2012
|
ORIGINAL ARTICLE
|
|
Comparative dosimetry of prostate brachytherapy with I-125 and Pd-103 seeds via SISCODES/MCNP* |
|
|
Autho(rs): Bruno Machado Trindade1; Marília Tavares Christóvão2; Daniela de Fátima Maia Trindade3; Patrícia Lima Falcão4; Tarcisio Passos Ribeiro de Campos5 |
|
|
Keywords: Radiotherapy; Dosimetry; Prostate cancer; Brachytherapy; SISCODES; MCNP. |
|
|
Abstract: INTRODUCTION
Brachytherapy is a safe and effective treatment for localized prostate cancer(1). Such technique utilizes sealed radioactive sources positioned adjacent to or in the interstitium of the cancerous tissue. As the absorbed dose is inversely proportional to the square of the distance from the emitting source, brachytherapy allows for the safe application of high absorbed doses at a determined target over a short period of time(2). According to the space where brachytherapy is applied, it can be classified as follows: intracavitary brachytherapy, involving insertion of radiation sources into natural body cavities; interstitial brachytherapy, insertion of the radioactive sources into the target tissue; intraluminal or intravascular brachytherapy, where the radiation sources are placed in the lumen of the esophagus, bronchi or blood vessels; and superficial brachytherapy, as the source is directly positioned on the skin or mucosa of the area to be treated(2). Also, it can be classified as temporary or permanent. On the report no.38 of the International Commission on Radiation Units (ICRU)(3), brachytherapy was classified, according to the dose rate, as low dose rate (LDR), medium dose rate (MDR) and high dose rate (HDR) for dose rates of 0.4 to 2 Gy.h-1; 2 to 12 Gy.h-1; and > 12 Gy.h-1, respectively. Interstitial brachytherapy with permanent implants of radioactive seeds is one of the methods utilized for treatment of prostate tumors, in general applied for neoplasms at T1 or T2 stages, with Gleason scores between 2 and 6, and serum PSA < 10 ng. The indications for implants are mostly for small volumes, particularly at early stages of disease located within the prostatic capsule (T1B-T1C, T2A-T2B-T2C stages, and absence of metastasis and lymphatic compromise). Such technique may be associated with teletherapy, particularly in cases of patients considered as being at high risk(4). Prostate brachytherapy primarily utilizes metal seeds of Iodine-125 (I-125) or, most recently, Palladium-103 (Pd-103)(5). Seeds of I-125 and Pd-103 are constituted of material doped with radioactive isotope encapsulated in a titanium casing. The external dimensions of the seeds are 4.5 to 5 mm in length, and 0.8 mm in diameter(6,7). Such seeds are represented on Figure 1. 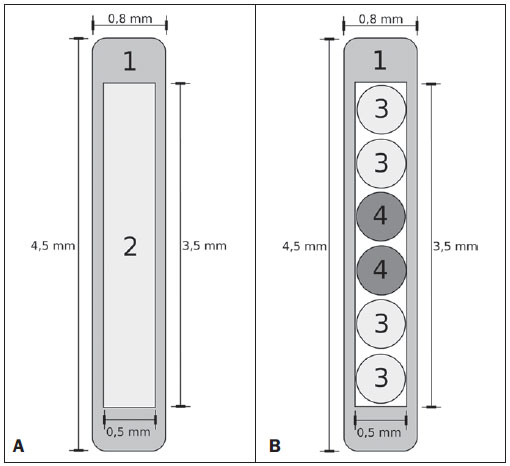 Figure 1. Diagram of I-125 (A) and Pd-103 (B) seeds. The seeds are composed of: 1 - titanium capsule; 2 - iodine oxide; 3 - Pd-103 doped polystyrene; 4 - Metal markers for the visualization of the seeds on X-ray images. I-125 is a radioactive isotope with a half-life of 60.1 days, emitting gamma rays some of which are internally converted into X-rays. The main photon emission energies are 27.20 keV, 27.47 keV and 30.97 keV, with the respective probabilities of 39.80%, 74.20% and 25.70%. On its turn, Pd-103 has a 17-day half-life and X-ray photon emission at the main energies of 20.07 keV, 20.22 keV and 22.71 keV, with probabilities of 19.83%, 37.55% and 11.88%, respectively(8). Because of their lower energies, Pd-103 photons have a lower range in the tissue than those emitted by I-125. Thus, for a same distribution of seeds with equal activity, the spatial dose distribution obtained by Pd-103 theoretically tends to be less homogeneous that that obtained with I-125. On the other hand, and for the same reason, Pd-103 seeds will produce lower absorbed doses at longer distances, particularly in the surrounding healthy tissues. The Pd-103 dose rate as well as its relative biological effectiveness (RBE) is higher than that of I-125(9). Thus, the prescription dose for Pb-103 seeds is lower than the recommended dose for I-125 seed implants, 125 and 144 Gy, respectively(10,11). The value corresponding to 80% of the dose deposited by I-125 seeds is delivered in 140 days, while the Pd-103 seeds deliver such value in 39 days. This is due to the fact that Pd-103 seeds generate higher dose rate and, consequently, produce a greater biological effect, particularly on cancer strains with a high proliferation index. The number of seeds implanted in the prostate depends upon the following parameters: reference volume to be irradiated, individual seeds activity, adopted prescription dose, as well as the selected model of seeds spatial distribution(12). The seeds are applied in an aligned pattern, by means of ultrasonography-guided insertion of needles containing a set of seeds. Such seeds are deployed on a one-by-one basis on the tissue, with a predetermined space between each other. The number and spatial distribution of the seeds are selected in such a manner to meet the prescription dose to the prostate, at the same time minimizing the dose delivered to adjacent tissues and structures. Obviously, there is a strong dependence between the management of the disease and the applied doses and also between applied doses and the arrangement of the seeds in the implant. In order to measure the treatment effectiveness, as well as the possibility of adverse effects, reference or merit parameters related to absorbed dose, are established for comparison purposes. For example, the Dx parameter corresponds to the minimum dose value in x% of an irradiated volume. Such volume may refer to an organ or to a predefined volume of interest in the radiotherapy planning. On its turn, the Dycc parameter corresponds to the minimum dose in the volume of y cm3 containing the highest absorbed dose in an organ or volume of interest defined in the planning(13). The Monte Carlo method is a mathematical technique applied in the reproduction of a statistical process. Such technique is particularly interesting in the resolution of complex problems which cannot be modeled by deterministic computational methods. The Monte Carlo N-Particle (MNCP) code applies such technique in the transport of nuclear particles, tracking each one of the many primary or secondary particles generated by a radioactive source, from the emission of such particles to some terminal event such as absorption, annihilation, escape from the system, among others(14). The three dimensional simulation of the nuclear particle transport is aimed at eliminating the two dimensional planning deficiencies by means of an analytical method in homogeneous medium, constituting an important tool to improve the quality of radiotherapy procedures in on cology(15,16). Computational methods have been highly relevant for dosimetric evaluation utilizing heterogeneous models(17-20). The SISCODES (Computational System for Dosimetry by Neutrons and Photons by Stochastic Methods) is a tool for the construction of computational simulation objects and simulation of radiotherapy treatments by means of stochastic codes such as MCNP(21,22). Such system allows the conversion of tomographic images into a voxel model. Based on a database including tissue chemical composition and nuclear data, SISCODES allows the association of nuclear and chemical data with the model of voxels by selection of the tissue of each voxel, as well as the positioning of brachytherapy and teletherapy sources. The system utilizes the MCNP for the simulation of nuclear particles transport in the model, consequently obtaining the absorbed dose. From the results, relevant dosimetry data are extracted and presented as spatial dose distribution and dose versus volume histogram (DVH)(21,22). The present article is aimed at investigating the dosimetry in prostate permanent implants of I-125 and Pd-103 seeds, by means of DVHs, isodoses and reference parameters, such as Dx and Dycc, with the purpose of performing comparisons between the two protocols. MATERIALS AND METHODS A protocol of permanent prostate implant of I-125 seeds was arbitrarily adopted. The same implant protocol was reproduced with Pd-103 seeds. The distribution and number of seeds were not altered between the protocols. The I-125 seeds were simply replaced by the Pd-103 ones. For the simulation of nuclear particle transport, a voxels model of a male pelvis(23) was utilized, with voxels of 5 x 5 x 5 mm. In such a model, the prostate volume corresponds to approximately 40 cm3. Figure 2 shows two sections of such a model. The seeds were positioned according to the modified uniform distribution proposed by Butler et al.(24), as shown on Figure 3. In total, 148 seeds were positioned in 24 applications, forming parallel lined-up segments in tangential direction to the pubic bones, on a spacing grid between segments of 1 cm, maintaining the seed spacing on a single segment of 6 mm between the centers of consecutive seeds. 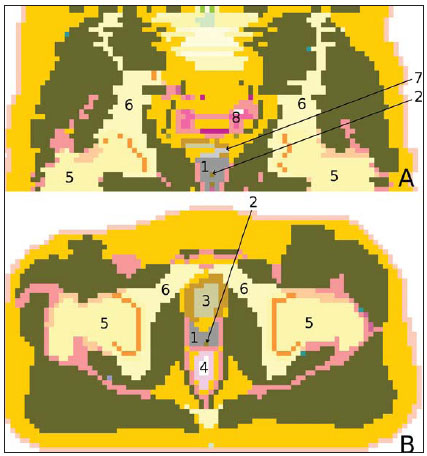 Figure 2. Frontal (A) and radial (B) sections of the voxel model, where the following anatomical structures can be observed: 1 -prostate; 2 - urethra; 3 - bladder; 4 - rectum; 5 - femur; 6 - iliac bones; 7 - seminal vesicle; 8 - colon. 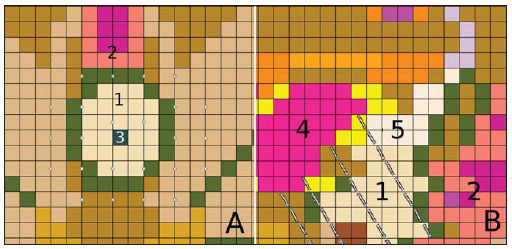 Figure 3. Section demonstrating the model with the seeds on the Z axis (A) (central view) and X axis (B) (sagittal view). The marked organs are the following: 1 - prostate; 2 - rectum; 3 - urethra; 4 - bladder; 5 - seminal vesicle. Both seeds types were represented by a cylinder of radioactive material measuring 3.5 mm in length and 0.5 mm in diameter contained inside another titanium cylinder with 5 mm in length and 0.8 mm in diameter, as shown on Figure 4. The radioactive matrix adopted for the I-125 seed was iodine oxide, and for the palladium seed, polystyrene doped with Pd-103. 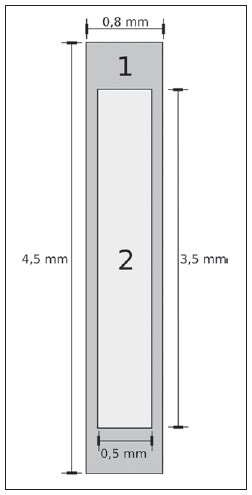 Figure 4. Model of the seed utilized at MCNP. The volume marked as 1 corresponds to the titanium capsule. The volume marked as 2 corresponds to the radioactive compound - iodine oxide or Pd-103 doped polystyrene. After simulation of each protocol with the MCNP (version 5.2), the results were imported by the SISCODES code. Then, the spatial dose distributions and DVHs of each procedure were generated. The initial activity of the seeds was calculated in order to obtain the prescription dose to the prostate recommended for each implant. The SISCODES code works as an interface for the MCNP code. The whole evaluation and simulation of nuclear particles transport is carried out by the MCNP code. Consequently, dosimetry is evaluated by MCNP. The SISCODES allows for the definition of the model and protocol to be simulated, and helps in the manipulation and presentation of the results as charts and histograms. The particle transport code calculates the deposited energy per mass unit for each voxel, in MeV.g-1 units per particle emitted by the source. Such value is converted into Gy (J.kg-1) by particle unit emitted by the source. The emissions from the sources are evaluated as a function of activity (Bq = transformations.s-1) multiplied by the photon production rate per transition unit. The results from such calculations generate the dose in Gy for each voxel. In the present case, one adopted the same activity for all seeds. The seed activity was calculated so that the prescribed doses (D90) were 144 and 125 Gy, respectively for I125 and Pd-103. Thus, the activities were found in such a way to adjust D90 to the value equivalent to the prescribed dose in a clinical protocol. With such activities, the absorbed doses were evaluated in the target regions as well as in the adjacent tissues, utilizing representation of isodoses regions and DVHs. RESULTS In order for the D90 to be equal to the recommended dose, the initial activity of each seed was calculated to be 0.42 mCi and 0.94 mCi, for I-125 and Pd-103, respectively. The resulting DVH for the prostate is shown on Figure 5A. Considering such activities and the adopted seeds spatial distribution, the maximum absorbed dose in the urethra was approximately 90% of the prescribed dose for I-125 and 108% of the prescribed dose for Pd-103. The D2cc dose in the rectum was 30 Gy for I-125 and 29 Gy for Pd-103, as shown on the DVH represented at Figure 5B. The same dose (D2cc) for the bladder was 127Gy for I-125 and 189 Gy for Pd-105 (Figure 5C). The pelvic bones presented high doses on the soft tissue-bone interface, particularly with the I-125 seeds. With I-125 seeds, the D10 value for the pubic bones was 144 Gy and for the iliac bones, the value was 2.7 Gy. The Pd-103 seeds presented a D10 value of 66 Gy for pubic bones, and 165 mGy for iliac bones. The differences in spatial dose distribution can be observed on the representation of spatial dose distribution shown on Figure 6. 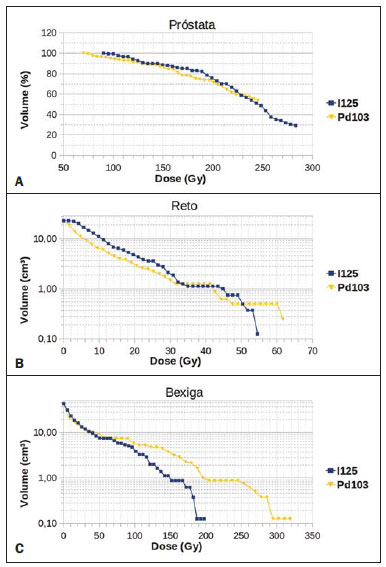 Figure 5. Dose volume histograms for the prostate, rectum and bladder. 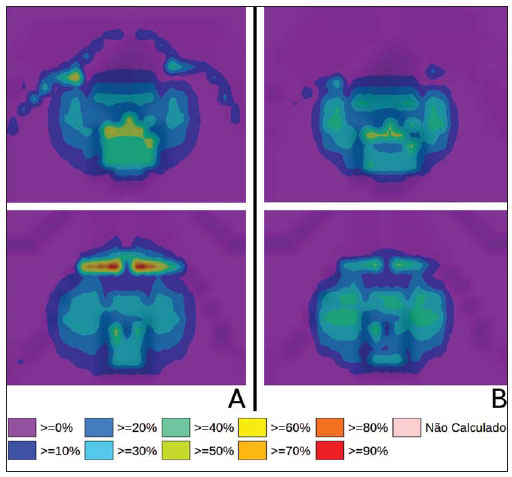 Figure 6. Spatial dose distribution in the prostate and surrounding tissues on two arbitrary axial views, for I-125 (A) and Pd-103 (B). The percentage on the caption refers to the maximum dose for each simulation. Observe the high doses in the soft tissue-bone interface, for the I-125 seeds, shown on the upper half of each image. Figure 6A shows high absorbed doses in the soft tissue-bone interface for the I125 seed implants. However, such high absorbed doses were not observed for Pd-103 seed implants (Figure 6B). The dose/volume ratios in the target volume (D90) with the dose in the rectum (D2cc) and in the bladder (D2cc) for the I-125 seeds implant were 0.208 and 0.882 respectively, while for Pd-103, the ratios were 0.232 and 1.512 respectively. The D90/D2cc dose ratio in the bladder and rectum for the Pd-103 seeds was higher than the one obtained with the I-125 seeds. DISCUSSION The following effects were taken into consideration in the present simulations: self-absorption in the matrix and in the metal casing of the seeds; interseed attenuation; tissue heterogeneity, including high densities in bone regions and present empty spaces; boundary effect and proximity with external air. This fact differentiates the method in the present article from the analytical calculations utilized for dosimetry in brachytherapy recommended by the AAPP Task Group 43, which considers an infinite homogeneous water-equivalent medium(10). The maximum absorbed dose in the urethra was below the threshold doses of the value D10 < 150% and D30 < 130% of the prescription dose with both seeds. The D2cc doses in the rectum were significantly lower than the threshold dose limit 145 Gy(24). The doses in the most irradiated volumes of the rectum, starting from D0.5cc, as well as in the bladder starting from D7cc, are higher with the Pd-103 seeds than with I125 seeds. This can be explained by the lower range of the Pd-103 photons, so that the energy is deposited on a smaller volume, increasing the absorbed dose in the seed surroundings. If a Pd-103 seed is close to one of the vital organs, the dose to this organ will be high. On the other hand, with the I-125 seed, the spatial dose distribution is more ample, but tending towards lower dose values per volume. Surprisingly, high doses in the order of 100% of the prescription dose were found in the interface between the pelvic bones and soft tissues. In such region, the doses from I-125 seeds are significantly higher than the doses from Pd-103 seeds, with D10 = 144 Gy and D10 = 66 Gy for I-125 and Pd-103, respectively. Such fact may also be explained by the greater range of I-125 photons, reaching the pelvic bones with higher intensity than those emitted by Pd-103. The effects of I-125 implants on such organs must be considered in clinical situations, requiring further investigation. It is important to observe that such effect is not observed in cases where the dosimetry is evaluated with analytical models and with homogeneous medium. CONCLUSIONS The Pd-103 and I-125 implants could deliver the prescription dose to the target volume. The Pd-103 implant is associated with a lower dose in pelvic bones, provided that the positioning of the seeds is carefully studied and that they are correctly positioned during the clinical protocol. The D2cc/D90 dose ratio in the rectum was 11.5% higher for Pd-103 seeds. In the studied case, the Pd-103 implants deposited a higher dose in the rectum and bladder as compared to I-125 implants. The main finding of the present study was the high absorbed doses outside the target region in bone tissues adjacent to the prostate, particularly in the case of I-125 implants. The computational methods utilized in the present study can be useful in future comparative clinical evaluations of brachytherapy with I-125 and Pd-103 seeds implants, among other radioactive seeds, and can be later validated by means of experimental trials simulating clinical protocols and dosimetry in realistic phantoms. Acknowledgements The authors wish to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for the support and assistance fin the investigations with the research group Núcleo de Radiações Ionizantes da Universidade Federal de Minas Gerais (NRI/UFMG). REFERENCES 1. Emami B. Oral cavity. In: Perez CA, Brady LW, editors. Principles and practice of radiation oncology. 3rd ed. Philadelphia, PA: Lippincott- Raven; 1998. p. 981-1002. 2. Williamson JF, Brenner DJ. Physics and biology of brachytherapy. In: Halperin EC, Perez CA, Brady LW, et al., editors. Perez and Brady's Principles and practice of radiation oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. p. 424-76. 3. International Commission on Radiation Units. Dose and volume specifications for reporting intracavitary therapy in gynecology. ICRU Report No. 38. Bethesda, MD: International Commission on Radiation Units; 1985. p. 1-16. 4. Chung HT, Speight JL, Roach M III. Intermediate and high-risk prostate cancer. In: Halperin EC, Perez CA, Brady LW, et al., editors. Perez and Brady's Principles and practice of radiation oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. p. 1484-503. 5. Zelefsky MJ, Valicenti RK, Hunt M, et al. Lowrisk prostate cancer. In: Halperin EC, Perez CA, Brady LW, et al., editors. Perez and Brady's Principles and practice of radiation oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. p. 1440-83. 6. Sowards K, Meigooni AS. A Monte Carlo evaluation of the dosimetric characteristics of the Best Model 2301 125I brachytherapy source. Appl Radiat Isot. 2002;57:327-33. 7. Li Z, Palta JR, Fan JJ. Monte Carlo calculations and experimental measurements of dosimetry parameters of a new 103Pd source. Med Phys. 2000;27:1108-12. 8. ENDF/B-VI Decay data. [acessado em 11 de novembro de 2011]. Disponível em: http://t2.lanl.gov/data/endf/decayVI.html 9. Ling CC, Li WX, Anderson LL. The relative biological effectiveness of I-125 and Pd-103. Int J Radiat Oncol Biol Phys. 1995;32:373-8. 10. Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys. 1995;22:209-34. 11. Beyer D, Nath R, Butler W, et al. American brachytherapy society recommendations for clinical implementation of NIST-1999 standards for (103)palladium brachytherapy. The clinical research committee of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2000;47:273-5. 12. Butler WM, Merrick GS, Lief JH, et al. Comparison of seed loading approaches in prostate brachytherapy. Med Phys. 2000;27:381-92. 13. Pötter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67-77. 14. X-5 Monte Carlo Team. MCNP - A general Monte Carlo N-Particle transport code, version 5. Los Alamos, NM: Los Alamos National Laboratory; 2003. 15. Purdy JA. Three-dimensional physics and treatment planning. In: Perez CA, Brady LW, editors. Principles and practice of radiation oncology. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998. p. 343-70. 16. Emami B, Graham MV, Michalski JM, et al. Three-dimensional conformal radiation therapy: clinical aspects. In: Perez CA, Brady LW, editors. Principles and practice of radiation oncology. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998. p.371-86. 17. Fonseca KR, Trindade BM, Campos TPR. Development of a voxel model of the heart for dosimetry. Artif Organs. 2011;35:459-64. 18. Brandão SF, Campos TPR. Brain tumour and infiltrations dosimetry of boron neutron capture therapy combined with 252Cf brachytherapy. Radiat Prot Dosimetry. 2012;149:289-96. 19. Christóvão MT, Campos TPR, Trindade BM. Simulation and dosimetric analysis of proton and carbon ion therapy in the treatment of uveal melanoma. Radiol Bras. 2011;44:367-73. 20. Campos TPR. Computational simulations in medical radiation. A new approach to improve therapy. [acessado em 11 de novembro de 2011]. Disponível em: http://www.sbmac.org.br/bol/boletim_2002/campos-4emc.pdf 21. Trindade BM, Campos TPR. Sistema computacional para dosimetria de nêutrons e fótons baseado em métodos estocásticos aplicado a radioterapia e radiologia. Radiol Bras. 2011;44:109-16. 22. Trindade BM. Remodelagem do sistema computacional para dosimetria em radioterapia por nêutrons e fótons baseado em métodos estocásticos - SISCODES. [tese de doutorado]. Belo Horizonte, MG: Universidade Federal de Minas Gerais; 2011. 23. Silva CHT. Desenvolvimento de fantomas: computacional de voxels e antropomórfico-antropométrico de pelve masculina para dosimetria em braquiterapia de próstata. [dissertação de mestrado]. Belo Horizonte, MG: Universidade Federal de Minas Gerais; 2004. 24. Salembier C, Lavagnini P, Nickers P, et al. Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother Oncol. 2007;83:3-10. 1. PhD, Postdoctoral Researcher, Núcleo de Radiações Ionizantes - Universidade Federal de Minas Gerais (NRI/UFMG), Belo Horizonte, MG, Brazil. 2. PhD, Technologist, Comissão Nacional de Energia Nuclear (CNEN), Belo Horizonte, MG, Brazil. 3. Master, Teacher, Centro Universitário Una, Belo Horizonte, MG, Brazil. 4. Postdoctoral Researcher, Núcleo de Radiações Ionizantes - Universidade Federal de Minas Gerais (NRI/UFMG), Belo Horizonte, MG, Brazil. 5. Postdoctoral Researcher, Associate Professor, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil. Mailing Address: Dr. Bruno Machado Trindade Rua Guilherme de Almeida, 255, ap.302, Santo Antônio Belo Horizonte, MG, Brazil, 30350-230 E-mail: bmtrindade@yahoo.com Received December 7, 2011. Accepted after revision August 27, 2012. * Study developed at Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554