Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 45 nº 5 - Set. / Out. of 2012
Vol. 45 nº 5 - Set. / Out. of 2012
|
ARTIGO ORIGINAL
|
|
|
|
|
Autho(rs): Alexandra Maria Vieira Monteiro1; Claudio Marcio Amaral de Oliveira Lima2; Paula Medina3 |
|
|
Descritores: Fluxo sanguíneo cerebral; Amamentação; Ultrassom Doppler; Neonatos. |
|
|
Resumo: INTRODUCTION
The cerebral adaptative mechanisms start developing in the intrauterine life. The fetus has different needs from the adult, and the cerebral blood flow supplies all the metabolic demands of the growth process (1-3). At birth, the cardiac output increases mostly to sustain overall systemic perfusions and the cerebral blood flow comprises a larger fraction, once the brain and body weight ratio is higher. At the first 2-34 hours of life, the ventricular outputs values significantly exceed those in older children and adults, and the cardiovascular system regulates the cerebral blood flow through variation in cardiac output(4). As the cerebral blood flow is evaluated, the resistive index remains increased at least until the first 12 hours(5), then progressively decreases with the gestational age and at each postnatal day(6-8). The cerebral blood flow supplies the brain's metabolic demand for O2 and glucose(9,10). Such mechanisms may be related to a myogenic process (arteriolar contraction or dilation), a neurogenic process (neural activity) or to metabolic demands, e.g., the relationship between the increase in of O2 levels and the cerebral demands(3,8,11-14). Usually, cranial Doppler ultrasonography is performed in babies in their mothers' lap, and during breastfeeding in order to warm and soothe babies. On the other hand, some studies including term infants diagnosed with mild or moderate hypoxicischemic encephalopathy at the neonatal period and with normal cranial ultrasonography have shown a relation between the resistance index and the neurological development in children until the first year of life(15). In such cases, as well as in others, it is important to take into account all the mechanisms which may influence the cerebral blood flow velocity, as breastfeeding. The present study was aimed at evaluating if there is any influence of breastfeeding on the cerebral blood flow velocities in healthy term neonates with normal cranial ultrasonography. MATERIALS AND METHODS The selection criteria for the present study were healthy babies with appropriate weight for the gestational age, postnatal age between 15 and 48 hours, born to mothers with no disease before or during pregnancy, whose deliveries showed no complications. The children were born in the University Hospital of Federal University of Rio de Janeiro (UFRJ). Out of the 256 included babies, 50.8% were female and 49.2%, male. The mean weight was 3,291 g (SD = 0.420), ranging from 2,250 to 4,635 g. On average, the mothers were 24.8 years old (SD = 6.1), ranging from 14 to 40 years. The Apgar score at the first minute ranged between 7 and 10 (mean 8.5; SD = 0.8); and at the fifth minute, between 8 and 10 (mean 9.3; SD = 0.5). The postnatal age ranged between 15 and 48 hours, with an average of 31.5 hours (SD = 9.1). The present study was approved by the Committee for Ethics in Research of the institution and all the parents signed a term of free and informed consent. All the infants had cranial ultrasonography with normal results performed just before the Doppler study. Examinations were performed with a real-time ultrasound scanner (Eccocee SSA 340A; Toshiba, Nasu, Japan) with a 3.5 MHz sector transducer. All the scans were performed with the babies in supine position, on their mother's lap, without eyes movement, during breastfeeding or resting before or after breastfeeding, through the anterior fontanelle, with sagittal sections on the anterior cerebral artery (ACA); coronal sections on the left middle cerebral artery (LMCA) and on the right middle cerebral artery (RMCA). The following Doppler parameters were measured: mean velocity (MV), resistance index (RI)(16) and pulsatility index (PI)(17) on the ACA, LMCA, and RMCA between the first 10 and 48 hours of life. The mean velocity (MV) is the area under velocity curve or velocity averaged. The RI, first described by Pourcelot (1976) is a blood-flow velocity waveform index, calculated by the following formula: RI = (PSV - EDV)/EDV where: PSV is the peak systolic velocity, EDV is the minimum forward diastolic velocity in unidirectional flow or the maximum negative velocity in diastolic flow reversal. The majority of investigators uses RI as indicative of cerebrovascular resistance because it minimizes the effect of transducer placement and can be easily obtained without velocity signal calibration. The PI is an arterial blood-flow velocity waveform index designed by Gosling et al. (1975) to quantify the pulsatility or oscillations of the waveform. Different definitions of this simplified PI may be found in the literature, but the following formula is most commonly used: PI = (PSV - EDV)/MV where: PSV is the peak systolic velocity, EDV is the end diastolic velocity or minimum forward diastolic velocity in unidirectional flow or the maximum negative velocity in diastolic flow reversal, and MV is the area under velocity curve or velocity averaged. The sample volume was 2, the filter, 100, and the depth, 10 cm. Statistical analysis was performed with the Epi-Info software. Data were analyzed by means of a paired t-test, Brieger's f-test for analysis of variance, and linear regression, with p < 0.01 being accepted as statistically significant. RESULTS The activities were grouped into "at rest" or "during breastfeeding", as informed by their mothers (see frequencies on Table 1). 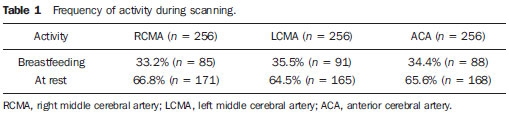 The relation between the activities and MV is shown on Figure 1; between the activities and pulsatility index, on Figure 2; between the activities and resistive index, on Figure 3. 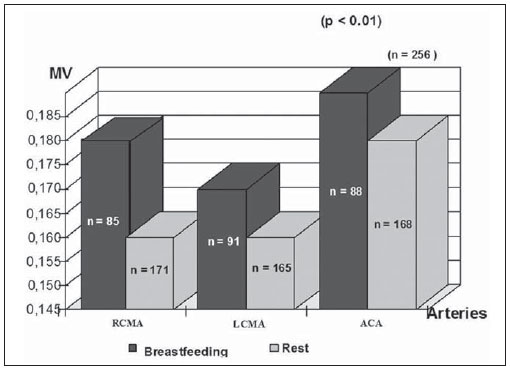 Figure 1. Relation between the activities and MV. (RCMA, right middle cerebral artery; LCMA, left middle cerebral artery; ACA, anterior cerebral artery; MV, mean velocity). 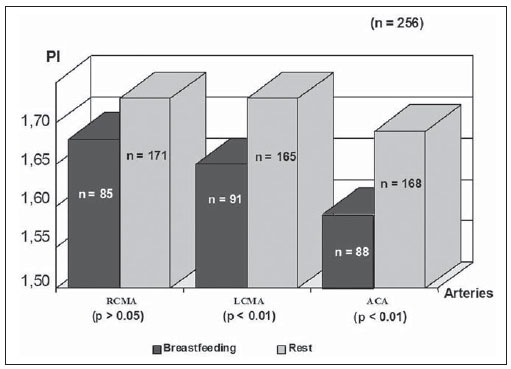 Figure 2. Relation between the activities and the PI. (RCMA, right middle cerebral artery; LCMA, left middle cerebral artery; ACA, anterior cerebral artery; PI, pulsatility index). 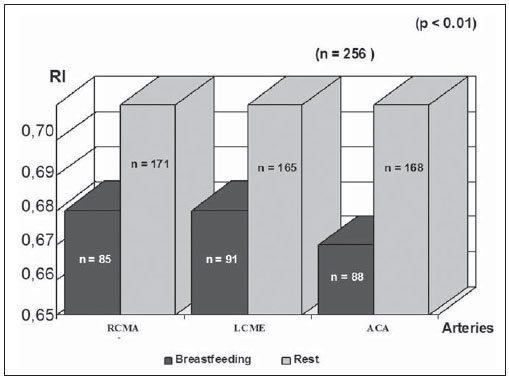 Figure 3. Relation between the activities and the RI. (RCMA, right middle cerebral artery; LCMA, left middle cerebral artery; ACA, anterior cerebral artery; RI, resistance index). All the studies presented statistical significance (p < 0.01) during breastfeeding, except on the RMCA, where p < 0.05. During breastfeeding, the authors observed the occurrence of an increase in MV and a decrease in PI and RI, which reverted to the pre-feeding level afterwards, probably due to the increase of the cerebral blood flow velocities caused by the sucking effort. DISCUSSION Ultrasonography has been widely used to evaluate the fetal brain(18-20). The effects of feeding on the cerebral blood flow were first considered by Hill et al.(21) who postulated in-balance between splanchnic and cranial flow. Since then, there have been a lot of related experimental studies, but rarely by using Doppler ultrasonography. The majority of studies involving Doppler ultrasonography in newborns and relating feeding and arterial blood flow velocities are concerned with the splanchnic haemodynamics adaptation to meal composition in normal full-term newborns(8,22- 25), the adaptation to the first feed(26-28) and the effect of enteral feeding on preterm newborns(29). Martinussen et al.(23) have described the circulatory responses before and after feeding in twenty healthy term infants within the first 24 hours, on the middle cerebral artery, by analyzing the Doppler parameters. They have noticed an increase in the cerebral blood flow velocities into the middle cerebral artery as a reflection of the increasing metabolic demand after feeding. In an experimental study developed by Gardiner(30) with calves, experiments were carried out on two groups of animals. Pedigree Jersey calves were obtained from local farms shortly after birth, and used at ages ranging from 2 to 29 days. Eighteen Suffolk lambs were used at ages ranging from 3 to 42 days. Gardiner has specifically investigated the direct effect of feeding on the cerebral blood flow and the oxygen consumption between 7 and 28 days after birth. He analyzed the cerebral blood flow five minutes prior to the feeding, at the second minute during the feeding, and at 6 and 30 minutes from the beginning. They have concluded that the cerebral blood flow increased during feeding and reverted afterwards to the pre-feeding level, while the heart rate reverted to the pre-feeding value around 1 minute after feeding stopped. Kusaka et al.(4) have also reported, in a experimental study, a positive relation between cardiac output and cerebral blood flow velocities in infants, using multichannel NIRS and pulse dye densitometry to demonstrate that the metabolic demand really influences the cerebral blood flow(29). The results of this original study with noninvasive cranial Doppler ultrasonography, including 256 healthy neonates to establish the influence of breastfeeding on the cerebral blood flow velocities, showed the occurrence, during breastfeeding, of an increase in MV and a decrease in the resistive index and pulsatility index as compared with values at rest, probably due to the increase in the cardiac output caused by the sucking effort, in agreement with other related studies(8,26-28). To the authors' knowledge, until now there is no normative study in the literature on Doppler ultrasonography parameters and approaching the influence of breastfeeding, although the authors usually utilize Doppler US in the follow-up, particularly in asphyxiated neonates. A study(15) including 20 term newborns with mild or moderate hypoxic-ischemic encephalopathy, high values of resistance index at the first examination, and without cerebral morphologic abnormalities or other diseases, was developed, evaluating the relation between RI at Doppler ultrasonography and clinical neurodevelopment. In such study, Doppler ultrasonography measurements were performed every two months as from the seventh day of life and the clinical neurodevelopment assessment, monthly up to one year of life. A progressive normalization of RI was observed in correlation with normalization of the neurologic abnormalities, but the activity of the child during each Doppler study has not been mentioned. The present study has proved a direct relation between the act of breastfeeding and the Doppler parameters values so such finding should be taken into account in Doppler measurements. It is the authors' opinion that the knowledge of newborns' cerebral hemodynamic profiles can contribute to an accurate interpretation of abnormal cranial Doppler findings in the analysis of pathologic flow velocities. REFERENCES 1. Volpe JJ, Perlman JM, Hill A, et al. Cerebral blood flow velocity in the human newborn: the value of its determination. Pediatrics. 1982;70:147-52. 2. Hallacoglu B, Sassaroli A, Fantini S, et al. Cerebral perfusion and oxygenation are impaired by folate deficiency in rat: absolute measurements with noninvasive near-infrared spectroscopy. J Cereb Blood Flow Metab. 2011;31:1482-92. 3. Geneslaw AS, Zhao M, Ma H, et al. Tissue hypoxia correlates with intensity of interictal spikes. J Cereb Blood Flow Metab. 2011;31:1394-402. 4. Kusaka T, Okubo K, Nagano K, et al. Cerebral distribution of cardiac output in newborn infants. Arch Dis Child Fetal Neonatal Ed. 2005;90:F77-8. 5. Deeg KH, Rupprecht T. Pulsed Doppler sonographic measurement of normal values for the flow velocities in the intracranial arteries of healthy newborns. Pediatr Radiol. 1989;19:71-8. 6. Pezzati M, Dani C, Biadaioli R, et al. Early postnatal doppler assessment of cerebral blood flow velocity in healthy preterm and term infants. Dev Med Child Neurol. 2002;44:745-52. 7. Allison JW, Faddis LA, Kinder DL, et al. Intracranial resistive index (RI) values in normal term infants during the first day of life. Pediatr Radiol. 2000;30:618-20. 8. Moron AF, Milani HJF, Barreto EQS, et al. Análise da reprodutibilidade do Doppler de amplitude tridimensional na avaliação da circulação do cérebro fetal. Radiol Bras. 2010;43:369-74. 9. Simpson DM, Infantosi AFC, Rosas DAB. Estimation and significance testing of cross-correlation between cerebral blood flow velocity and background electroencephalograph activity in signals with missing samples. Med Biol Eng Comput. 2001;39:428-33. 10. Wilson MH, Edsell MEG, Davagnanam I, et al. Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia - an ultrasound and MRI study. J Cereb Blood Flow Metab. 2011;31:2019-29. 11. Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol. 1984;101:161-211. 12. Mchedlishvili G. Physiological mechanisms controlling cerebral blood flow. Stroke. 1980;11:240-8. 13. Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161-92. 14. Daffertshofer M, Hennerici M. Cerebrovascular regulation and vasoneuronal coupling. J Clin Ultrasound. 1995;23:125-38. 15. Garcia MHM, Monteiro AMV, Freire SM. Relation between the resistance index obtained by the transfontanellar Doppler ultrasonography and the neurological development until the first year of life in term infants with mild or moderate hypoxic- ischaemic encephalopathy. Arq Neuropsiquiatr. 2007;65:1206-10. 16. Pourcelot L. Diagnostic ultrasound for cerebral vascular diseases. In: Donaldi J, Levis S, editors. Present and future of diagnostic ultrasound. Rotterdam, Netherlands: Kooyker; 1976. p. 141-7. 17. Gosling RG, King DH. Ultrasound angiology. In: Harcus AW, Adamson J, editors. Arteries and veins. 1st ed. Edinburgh: Churchill-Livingstone; 1975. p. 61-98. 18. Garcia RF, Lederman HM, Brandão J. Estudo dos ventrículos cerebrais por ultrassonografia, na criança normal, nascida a termo, de 1 a 6 meses. Radiol Bras. 2011;44:349-54. 19. Gabriel ML, Piatto VB, Souza AS. Aplicação clínica da ultrassonografia craniana com Doppler em neonatos prematuros de muito baixo peso. Radiol Bras. 2010;43:213-8. 20. Monteiro AMV, Lima CMAO, Ribeiro EB, et al. Diagnóstico por imagem e aspectos clínicos da trombose venosa cerebral em recém-natos a termo sem dano cerebral: revisão em 10 anos. Radiol Bras. 2010;43:149-53. 21. Hill PD, Aldag JC, Chatterton RT Jr. Breastfeeding experience and milk weight in lactating mothers pumping for preterm infants. Birth. 1999;26:233-8. 22. Hsu CH, Lee HC, Huang FY. Duplex ultrasonographic assessment of gut blood flow velocity: effect of meal composition in normal full-term newborns after first feed. J Ultrasound Med. 1994;13:15-8. 23. Ozkan H, Oren H, Erdag N, et al. Breast milk versus infant formulas: effects on intestinal blood flow in neonates. Indian J Pediatr. 1994;61:703-9. 24. Martinussen M, Brubakk AM, Linker DT, et al. Mesenteric blood flow velocity and its relation to circulatory adaptation during the first week of life in healthy term infants. Pediatr Res. 1994;36:334-9. 25. Robel-Tillig E, Möckel A, Vogtmann C. Normal Doppler ultrasound values of the anterior cerebral artery of premature and newborn infants with reference to cardiac function parameters and intestinal blood flow profile. Z Geburtshilfe Neonatol. 1999;203:234-40. 26. Van Bel F, Van Zwieten PH, Guit GL, et al. Superior mesenteric artery blood flow velocity and estimated volume flow: duplex Doppler US study of preterm and term neonates. Radiology, 1990;174:165-9. 27. Coombs RC, Morgan ME, Durbin GM, et al. Doppler assessment of human neonatal gut blood flow velocities: postnatal adaptation and response to feeds. J Pediatr Gastroenterol Nutr. 1992;15:6- 12. 28. Aranha CA, Lederman HM, Segre CAM. Color Doppler evaluation of the influence of type of delivery, sex, postnatal age and time post feeding on full term healthy newborns cerebral blood flow. Arq Neuropsiquiatr. 2009;67:463-73. 29. Gladman G, Sims DG, Chiswick ML. Gastrointestinal blood flow velocity after the first feed. Arch Dis Child. 1991;66(1 Spec No):17-20. 30. Gardiner RM. Cerebral blood flow and oxidative metabolism during hypoxia and asphyxia in the new-born calf and lamb. J Physiol. 1980;305:357-76. 1. PhD, Associate Professor, Department of Radiology, Medical School, State University of Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil. 2. MD, Radiologist, Fellow degree, Department of Radiology, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. 3. Undergraduate Student, Medical School, State University of Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil. Mailing Address: Dra. Alexandra Monteiro Avenida Vinte e Oito de Setembro,77, térreo, sala 126, Vila Isabel Rio de Janeiro, RJ, Brazil, 20551-030. E-mail: monteiroamv@gmail.com / cmaolima@gmail.com Received May 10, 2012. Accepted after revision August 17, 2012. * Study developed at Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554