Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 45 nº 5 - Sep. / Oct. of 2012
Vol. 45 nº 5 - Sep. / Oct. of 2012
|
ORIGINAL ARTICLE
|
|
Endovascular treatment of thoracic aortic diseases: a single center result analysis* |
|
|
Autho(rs): Eduardo Rafael Novero1; Patrick Bastos Metzger2; Javier Obregon1; Vanessa Luciene Abreu de Marco3; Fabio Henrique Rossi4; Samuel Martins Moreira5; Nilo Mitsuru Izukawa6; Antonio Massamitsu Kambara7 |
|
|
Keywords: Aneurysm; Vascular graft; Atherosclerosis. |
|
|
Abstract: INTRODUCTION
Thoracic aortic aneurysm (TAA) is among the 15 leading causes of death the United States. Only 39% of the patients with untreated TAA survive five years. Patients with TAA with more than 8 cm in diameter present 80% of risk for rupture and death within one year of follow-up. The aneurysm diameter is the most relevant factor in the determination of such risk(1,2). Aortic dissection is the most feared condition of the aorta and is twice as frequent as aortic rupture. The mortality rate associated with dissection ranges between 1% and 2% per hour, during the first 48 hours. Risk factors frequently associated with such condition include hypertension, cardiovascular and respiratory diseases. Dissection is three times more frequent in men than in women. The most common entry tear sites are the ascending aorta, some centimeters distal from the aortic valve, and the descending aorta, below the origin of the subclavian artery. Acute dissection differentiates from chronic dissection in cases where the diagnosis is established within 14 days from the symptoms onset. In cases where a type B dissection progresses with ischemic complications, surgical or endovascular intervention is mandatory. Approximately 30% of patients with type B dissection develop complications. The mortality associated with surgical correction of type B acute dissection ranges from 30% to 80% in cases of renal or mesenteric ischemia. Endovascular therapy can offer techniques of reperfusion of ischemic vascular beds in complicated dissections, with lower risks than those in open surgery(3). Aortic dissection and TAAs represent conditions with sometimes catastrophic consequences. Despite the developments in diagnostic methods, operative techniques and intra- and postoperative management, such conditions still constitute major challenges for endovascular surgeons(4). The concept that aortic diseases could be corrected by endoluminal prostheses positioned within the aorta by means of catheters inserted through the femoral artery has emerged over the past few years. As the high morbimortality rate related to surgical interventions in cases of thoracic aneurysm dissection is considered, the endovascular technique becomes a very attractive option. Endovascular devices allow for a minimally invasive approach, with the flow being maintained without clamping(5). Other advantages include lower blood transfusion rate, shorter hospital stay, shorter intensive care unit (ICU) stay and lower costs. The main disadvantage is that such a technique predisposes the patient to a higher number of reinterventions in the medium and long terms(4). The endovascular intervention involving the thoracic aorta has been poorly compared with open surgery in controlled and randomized clinical studies, but medium and long term post-implantation results have been encouraging, with lower rates of early morbimortality as compared with open surgery.(6) According to some authors, the endovascular treatment of true TAAs poses a greater challenge than that performed in patients with chronic type B dissection, with lower rates of therapeutic success and higher mortality(6,7). The present study is aimed at evaluating the clinical results obtained with a consecutive series of patients submitted to endovascular correction of TAAs and chronic type B aortic dissection, analyzing the technical and therapeutic success, morbimortality, complications and reintervention rate. MATERIALS AND METHODS The present retrospective, longitudinal and observational study was developed in a reference center for cardiovascular disorders, in the period between January, 2010 and July, 2011, evaluating 55 patients submitted endovascular repair of thoracic aorta. Such a population was divided into two groups, as follows: group 1 (G1) - patients with true TAA, penetrating ulcer of thoracic aorta and pseudoaneurysm; group 2 (G2) - patients with type B aortic dissection and intramural hematoma. Both groups included patients whose indication for endovascular procedure was treatment of leakage of previous endoprosthesis, independently of the underlying disease. The present study included both male and female patients, with or without thoracic symptoms, with indication of aortic correction for: a) TAA with > 55 mm in diameter, correction of type I or type III leakage; b) chronic type B dissection with aneurysmal dilation > 55 mm or correction of type I or III leakage; c) thoracic aorta pseudoaneurysm; d) penetrating ulcer of thoracic aorta with > 2 cm in diameter and 1 cm in depth. Exclusion criteria were the following: patients with proximal aortic neck with < 15 mm in length; distal aortic neck with < 15 mm in length from the celiac trunk; presence of thrombus or calcifications with > 50% of the neck diameter; external iliac arteries with < 7 mm in diameter; serum creatinine levels > 2.0 mg/dl or creatinine clearance < 30 ml/min. Those patients who remained in the present study population according to the inclusion and exclusion criteria, were considered as having a favorable anatomy and were referred to endovascular treatment. The evaluation of cardiac and/or anesthetic risks assessments was not taken into consideration for inclusion/exclusion of patients. In all of the cases, the diagnosis and the therapeutic schedule were based on computed tomography angiography, and the role of preoperative arteriography remained as an optional diagnostic method. Computed tomography images reconstruction was performed with the aid of the Osirix® software with 3D viewer and multiplanar reconstruction (MPR) modes in order to obtain diameter, angle and length of the aneurysms proximal and distal aortic necks (Figure 1). 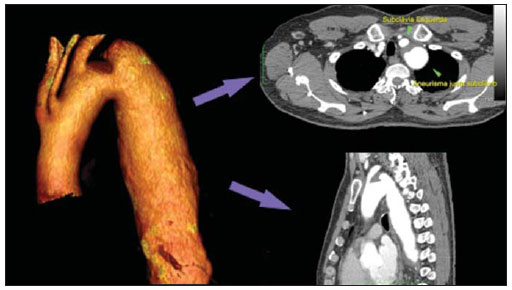 Figure 1. Computed tomography angiography with 3D reformation, and axial and sagittal sections. Thoracic juxta-subclavian aneurysm in type 3 aortic arch. Surgical technique All procedures were performed in the hemodynamics laboratory of the Centro de Intervenções Endovasculares (CIEV) of Instituto Dante Pazzanese de Cardiologia, in São Paulo, SP, Brazil. The patients were treated under general anesthesia by inhalation. The antimicrobial prophylaxis was performed with 1.5 g of cefuroxime at the moment of anesthesia induction. The preferred approach was through the common femoral artery with uni- or bilateral open surgical dissection, according to the selected type of intervention or endoprosthesis. In case of unfeasibility of such an approach, the external iliac artery by retroperitoneal access was utilized as an alternative. The radiographic control was performed with a Siemens Artis Flat Panel apparatus. The following devices were utilized: Valiant® (Medtronic; Minneapolis, MN, USA), Zenith® TX2® (Cook Medical; Bloomington, IN, USA), TAG® (Gore Medical; Flagstaff, AZ, USA), HerculesTM (MicroPort; Shangai, China), Relay® (Bolton Medical; Sunrise, FL, USA), and E®-vita (Jotec GmbH; Hechingen, Germany). Intraoperative arteriography was performed in all the patients. The immediate postoperative follow-up was undertaken in the ICU in all the cases (Figure 2). 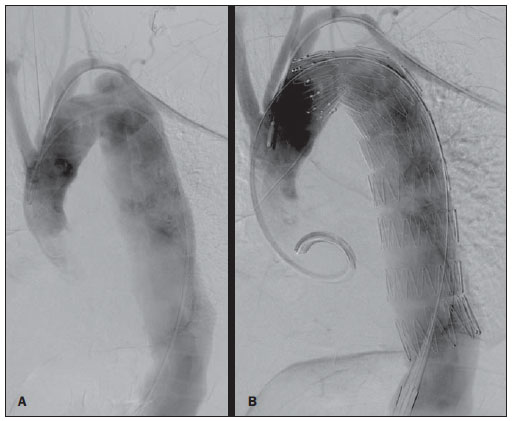 Figure 2. Intraprocedural aortography by left brachial approach (A) and final outcome of endovascular repair of thoracic aorta aneurysm (B). Absence of intraoperative leakage and preservation of left subclavian artery. All the individuals were postoperatively followed-up on an outpatient basis 15, 30, 180 and 360 days after the procedure. After the first year, the follow-up was performed once a year. Computed tomography angiography was performed at the 30th and 360th days of follow-up. The analyzed outcomes were defined as per the following description: Primary outcomes 1. Technical success - when the objective of deploying the endoprosthesis in the affected area was achieved, either with or without the presence of leakage or other complications that might negatively influence the progression of the aortic disease. 2. Therapeutic success - when the deployment of the endoprosthesis occurred without leakage or other complications that might affect the favorable course of the aortic disease. 3. Perioperative mortality - number of deaths recorded within 30 days after the procedure. 4. Complications - a) intraoperative complications: those occurred in the hemodynamics room during the intervention; b) intra-hospital complications: those occurred during hospital stay, out of the hemodynamics room and within 30 days after the intervention. The following events were considered as being complications: local bleeding; presence of inguinal or retroperitoneal hematoma; inadvertent occlusion of the subclavian artery during the device deployment; presence of peripheral embolization of lower limbs as a cause of acute arterial occlusion; occurrence of paraplegia or paraparesis; infections, either in the surgical site, lower respiratory tract or in the utilized endoprosthesis; development of acute renal failure, defined as an increase in creatinine levels greater than twice the value observed previously to the procedure; and death. 5. Reintervention rate - interventions performed for maintenance of appropriate functioning of the endoprosthesis or for resolution of complications associated with the intervention. Secondary outcomes 1. Hospitalization time - time span between admission and hospital discharge. 2. Procedural time- elapsed time between the start of anesthetic induction and the closure of the operative site. Leakages 1. Initial or primary leakages - those originated during the initial procedure or those diagnosed within the first 30 postoperative days. 2. Secondary leakages - those diagnosed after 30 days from the initial procedure. Definitions Carotid-subclavian and/or carotid-carotid shunt to extra-anatomic bypass graft performed for anchoring the endoprosthesis in zones 1 and 2, in cases of compromise of the left carotid or left subclavian arteries. RESULTS The present series included 55 patients submitted to correction of thoracic aortic diseases. Group 1 comprised 29 patients. The primary diseases in this population were true TAAs in 26 cases (89.6%), penetrating ulcer in 2 cases (6.8%), and pseudoaneurysm in 1 case (3.4%). The mean age was 66.8% ± 10 years, and 18 patients were men (69%). The patients were asymptomatic in 20 cases (68.9%) and were incidentally diagnosed with basis on findings at routine examinations. The predominant risk factor was hypertension in 28 patients (96.8%), 19 patients were smokers (65.5%) and 12 (41.3%) presented dyslipidemia. Diabetes was present in 4 cases (13.7%). Obesity, with body mass index > 40, was present in 2 patients (6.89%) of the population (Table 1). Among the present comorbidities, the authors have found a high incidence of ischemic cardiopathy (58.6%), acute renal failure (17.2%) and, in lesser degree, dilated cardiopathy in one case (3.4%). Also, a significant percentage (20.6%) of patients previously submitted to endovascular aortic aneurysm correction was observed. Chronic obstructive pulmonary disease was present in two cases (6.9%) (Table 2). 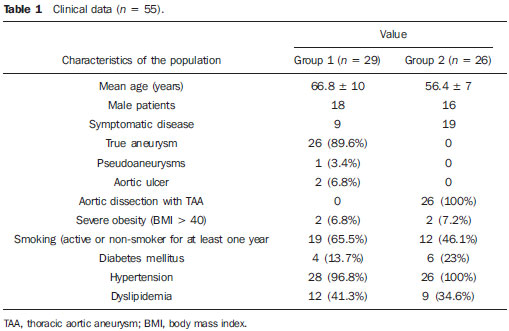 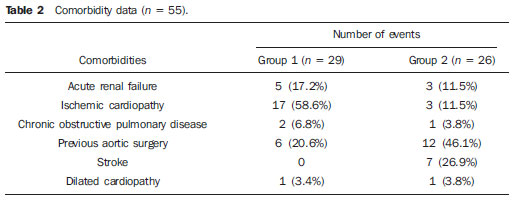 The indications for endovascular treatment in this population were distributed as follows: true TAAs in 23 cases (79.3%), leakage in previously implanted endoprosthesis in 3 cases (10.3%), penetrating ulcer in 2 cases (6.8%), and pseudoaneurysm in 1 case (3.4%). Among the treated aneurysms, 17 were thoracic (10 saccular, and 7 fusiform), and 9 were thoracoabdominal (3 cases were type I, 3 type II, 2 type III and 1 case was type V, according to the Saffi classification). The treatment was elective in all of the cases. Inhalation anesthesia was utilized in all cases, with liquor drainage in those patients with indication for aortic reintervention, in those with previous abdominal or thoracic aortic surgery, and in those patients with preoperatively documented visceral or supra-aortic trunk occlusion. Technical success was obtained in 86.2% of the cases, i.e. in 25 patients it was possible to deploy the endoprosthesis in the desired location. In four cases, the endoprosthesis could not reach the desired location because of technical or anatomical difficulties (calcification or tortuosity). Therapeutic success was achieved in 58.6% of the cases, i.e., in 17 patients the endoprosthesis was deployed without leakage or other events that might negatively affect the outcome of the intervention. The only cause of therapeutic failure was leakage or its persistence, according to the indication for the intervention. The complication rate was 48.2%, with the following most frequent intra-operative complication: femoral lesion in 7 cases (24.5%) and peripheral embolism in 1 case (3.4%). Intra-hospital postoperative complications included infection in 2 cases (6.8%) - both surgical wound infection -, acute renal failure in 2 patients (6.8%), paraplegia in 1 case (3.4%), and retroperitoneal hematoma in 1 case (3.4%) (Table 3). 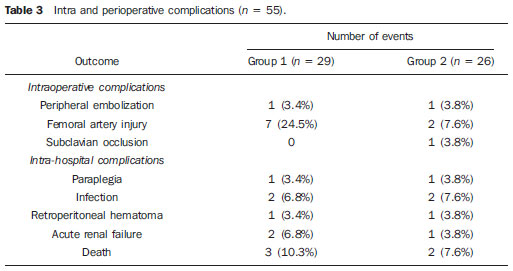 Perioperative mortality rate was 10.3%. One death occurred due to aneurysm rupture during the deployment of the endoprosthesis; two other deaths occurred as a consequence of sepsis by respiratory tract infection (Table 4). No death was observed 30 days after surgery. The total primary leakage rate was 27.5%, with type Ia leakage being observed in 4 cases, and type Ib in 3 cases. Type IV leakage was present in 1 case (3.4%). There was no case of type III leakage and endoprosthesis migration during follow-up of the patients. The rate of reinterventions within one year was 10.3%, due to treatment of type I leakage (Table 4). One-year survival during followup was 89.7%. One death occurred due vascular causes. 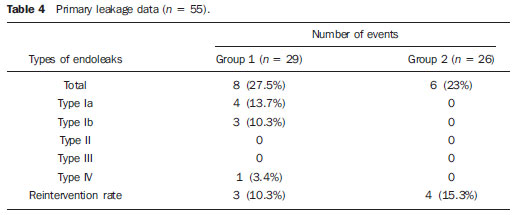 The mean procedure duration was 78 minutes (ranging from 54 to 115 minutes), and the mean hospitalization time was 4.2 days with a variation of 7 days. The most utilized endoprosthesis devices were the following: Valiant® in 13 cases (45%), Zenith® TX2® in 3 cases (10.4%), TAG® in 3 cases (10.4%), HerculesTM in 3 cases (10.4%) and E®-vita in 3 cases (10.4%). The rate of carotid-carotid shunt and/or carotid-subclavian shunt was 6.8%, i.e., 2 cases required such shunting. The endoprosthesis anchoring zones were distributed as follows: 25 cases (86.2%) in zone 3, two cases in zone 4 (6.9%), one in zone 2 and one in zone 1 (Figure 3). 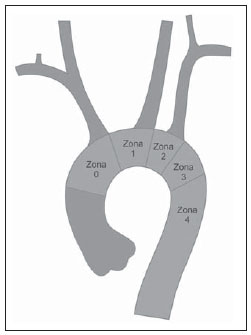 Figure 3. Endoprosthesis anchoring zones in thoracic aorta aneurysms. Group 2 included 26 patients treated for chronic type B aortic dissection. In this population, the primary condition was type B aortic dissection in 100% of the cases. The mean age was 56.4 ± 7 years, and 16 (61.5%) patients were men. The patients were symptomatic in 19 cases (73%), 2 cases (7.2%) presented nonspecific symptoms and 5 cases did not present any symptom (19.2%). Hypertension was the predominant risk factor in 26 patients (100%), 12 patients (46.1%) were smokers, and 9 patients (34.6%) presented dyslipidemia. Diabetes was present in 6 cases (23%). Obesity, with a body mass index > 40 was present in 2 patients (7.2%) of the population (Table 1). The following comorbidities were observed: high incidence of previous aortic surgeries, present in 12 cases (46.1%), stroke in 7 cases (26.9%), chronic renal failure in 3 cases (11.5%), and ischemic cardiopathy in 3 cases (11.5%), besides dilated cardiopathy, to a lesser degree, in 1 case (3.8%). Chronic obstructive pulmonary disease was present in 1 case (3.8%) (Table 2). The indications for endovascular treatment in this population were distributed as follows: type B dissection in 15 cases (57.6%) and leakage in previously implanted endoprosthesis in 11 cases (42.3%). In this group, all the patients were submitted to elective treatment. General inhalation anesthesia was utilized in all cases, with liquor drainage in those patients with indication for aortic reintervention, in those with previous history of thoracic or abdominal surgery, and in the patients who presented preoperatively documented visceral or supra-aortic trunk obstruction. The technical success reached 100%. The endoprostheses were deployed in the desired site in all cases. Therapeutic success was achieved in 74% of cases, i.e., in 20 patients the endoprosthesis was deployed without leakage or other events that might negatively affect the outcome of the intervention. The sole cause of therapeutic failure was leakage or its persistence, according to the indication for the intervention. The rate of complications was 30.7%, and the most frequent intra-operative complications were femoral lesion in 2 cases (7.6%) and involuntary occlusion of the subclavian artery in 1 case (3.8%). Intrahospital postoperative complications were the following: infections in 2 cases (7.6%), retroperitoneal hematoma in 1 case (3.8%), acute renal failure in 1 patient (3.8%), and paraplegia in 1 case (3.8%) (Table 3). The perioperative mortality rate was 7.6%. Two deaths occurred: one because of sepsis caused by to lower respiratory tract infection, and another caused by cardiopulmonary arrest secondary to Chagas-related dilated cardiopathy (Table 3). Three deaths occurred after 30 days from surgery, two because of recurrent dissection, and one because of acute pulmonary edema. The total rate of primary leakage was 23%, with type Ia leakage being observed in all such cases. Types II and III leakages as well as endoprosthesis migration were not observed during follow-up of such patients. The rate of reintervention within one year was 15.3%, as a result of type I leakage treatment (Table 4). The one-year survival rate during follow-up was 80.7%. The mean duration of procedures was 67 minutes (ranging between 49 and 104 minutes), and the mean hospitalization time was 9.9 days with a variation of 8 days. The most utilized endoprosthesis devices were the following: Valiant® in 10 cases (38.5%), Zenith® TX2® in 7 cases (27%), TAG® in 6 cases (23%), Relay® in 2 cases (7.7%), and HerculesTM in 1 case (3.8%). The rate of carotid-subclavian and/or carotid-carotid shunting was 23%, i.e., in 6 cases such shunt was necessary. The endoprosthesis anchoring zones were distributed as follows: 6 cases in zone 2 (23%), 14 cases (53.8%) in zone III, and 6 cases in zone 4 (23%) (Figure3). DISCUSSION Despite the recognition of its indication, the endovascular treatment of chronic aortic disease presents results that are variable and dependent upon the analyzed population(7). Physiopathological and anatomic differences between aneurysms and aortic dissections determine operative techniques translating into different results. Because of the clinical instability and weakness of the aortic wall in thoracic aortic dissection, the utilization of proximal compliant balloon after endoprosthesis implant is not recommended. On the other hand, in the case of aneurysms, the anatomic distortion translated into greater tortuosity of the aortic arch and higher incidence of stenosis by peripheral atherosclerotic plaques frequently limit the smooth advancement of the deployment system in this population. Brazilian authors have demonstrated a rate of technical success of 97% in 92 patients treated for true TAAs, and 98% in 130 patients treated for type B aortic dissection(7). The EUROSTAR study has demonstrated a rate of primary technical success of 89% in patients with aortic dissection(8). In the present study, the rate of technical success reached 86.2% for G1 and 100% for G2. In 4 cases, the devices could not be deployed in the descending thoracic aorta of patients with true aneurysms because of limitations of the aortic and iliacfemoral axis anatomy. The rate of therapeutic success was 58.6% for G1 and 73% for G2. Brazilian authors have presented a rate of clinical success of 71% in cases of aneurysm and 84% in cases of dissection(7). Type I leakage was the most frequent event associated with endovascular correction of aortic aneurysm and dissection and may eventually lead to clinical failure. The rates reported in the literature range from 0% to 44%(9,10). In the present study population, leakage was the only cause for therapeutic failure. The rate of primary leakage in G1 was 27.5%, while in G2, it was 23%. It is known that a proximal neck smaller than 2 cm in the left subclavian artery and the presence of an entry tear located on the small curvature of the aortic arch constitute predisposing factors of leakage and endoprosthesis kinking observed in the angiographic follow-up. The evolution of type I leakage is controversial. Some authors believe that there is necessity of deployment of an additional endoprosthesis, eventual embolization or surgical conversion. However, there are reports on spontaneous thrombosis without any other type of treatment, despite the initial presence leakage during the procedure. The period of spontaneous resolution ranges from one week to eight months. In case of persistence of such leakage, corrective therapeutic interventions must be performed(11). In the population with type I leakage, the authors found five patients in G1 and two in G2 with low-volume leakages at arteriography, which spontaneously resolved between the third and sixth months of follow-up. Two G1 patients presented neck length < 2 cm. Such data translates into better rates of therapeutic success without precipitated interventions, and also into smaller future reinterventions. No secondary leakage was observed in both groups. Considering the cases of spontaneous resolution, the annual rates of reinterventions were 10.3% for G1 and 15.3% for G2. The VALOR study has presented annual leakage rate of 17%, with type I leakage occurring in 6.3% of the cases, type II in 9.5%, and type III in 1.9%(12). In the Group1, three patients (10.3%) presented leakage as indication for endovascular procedure. Among them, one patient had a previous history of aortic surgery, and two had previously undergone endovascular treatment. Upon the analysis of the type of treated leakage, the author observed that two patients had type Ia leakage persisting after intervention, while one patient had type Ib leakage with therapeutic success after intervention. In the Group 2, 11 patients (42.3) had leakage as indication for the endovascular treatment; four of them with previous aortic surgical treatment and seven with previous endovascular treatment. As regards leakage types, seven cases were of type Ia, with therapeutic success in three of them and persistence of leakage in four patients; and four cases presented type Ib leakage, all of them being successfully corrected. The authors have observed that the rate of therapeutic success was lower in cases of type Ia leakage than in cases of type Ib leakage, a phenomenon that was more noticeable in G1. Thus, in G1, type Ia leakages could not be corrected, while in G2 only 43% of the leakages were corrected. In order to explain such data, the authors observed that, in G1, the percentage of endoprosthesis anchoring in zones 1 and 2 was 6.8%. In 6.8% of the patients carotidsubclavian and/or carotid-carotid shunt was performed, which allowed for endoprosthesis anchoring in zone 1 or 2 with greater safety, thus occluding the subclavian and/or the left common carotid arteries. In G2, 23% of the endoprostheses were anchored in zone 2. All such patients were submitted to carotid-subclavian shunting. Probably, the anchoring zone 2 was underutilized for correcting type Ia leakages in G1. Coverage of the subclavian artery is generally well tolerated and rarely the patients develop dizziness or left upper limb claudication. The VALOR study has demonstrated carotid-subclavian shunt in 5% of cases. Many groups are electively and belatedly submitted to such a shunting, in case symptoms manifest(12). Avoiding aortic clamping with the endovascular technique reduces the complications by ischemia of the target organ, as well as cardiac (1% versus 10%), respiratory (8% versus28%) and renal complications, as compared with open surgery(13). On the other hand, injury of the arterial access path caused by the combination of a high-profile delivery system (20-24 French) and iliac-femoral atherosclerosis is very frequently observed in procedures with the endovascular technique. In the VALOR study, the patients with true TAAs presented complications in 41% of cases(12). In the series published by the Arizona Heart Institute, mild complication occurred in only 38% of patients with dissection(14). The present study has found a complication rate of 48.2% in G1 and 30.7% in G2. The population treated for true TAAs shows femoral injury in 24.1% of cases, a rate considerably higher than in G2, with 7.6%. Peripheral atherosclerosis in this population plays a role not only in this type of injury, but also in the incidence of peripheral embolization caused by the prolonged manipulation of guidewires or of the device itself within the aortic arch. The endovascular correction of TAAs is associated with risk for medullary ischemia and permanent paraplegia. The main predictive factor is the extent of the aortic segment covered by the endoprosthesis(15). According to the Gore TAG protocol, which has compared open surgery versus endovascular treatment in TAAs, the incidence of paraplegia is lower in endovascular treatment (14% versus 3%)(16). Surgically treated dissections have a paraplegia incidence of 19%, but with the endovascular technique the incidence is around 0-10% (mean = 1.8%)(17-19). The EUROSTAR registry presents paraplegia incidence of 4% in patients treated for aneurysms, and 0.8% for aortic dissections treated with endovascular approach(8). In the analyzed population, two cases of permanent paraplegia were observed, as follows: one in G1 (3.4%) and another in G2 (3.8%). No case of stroke was observed in either group. Previous or simultaneous surgery, coverage of extensive aortic segment and perioperative hypotension represent risk factors for paraplegia after TAA correction. Prophylactic cerebrospinal fluid drainage may be useful in high-risk patients, i.e., those patients presenting associated TAA and abdominal aortic aneurysm, previous history of either open or endovascular abdominal aneurysm correction, need for iliac arterial conduit for endoprosthesis deployment in patients with a history of transient lower limb paralysis and in the impossibility of cerebrospinal fluid drainage in less than one hour after endoprosthesis deployment in cases where paraparesis or paraplegia are expected. The main disadvantage of the preoperative drainage is the potential development of spinal hematoma in cases where heparin is administered during the intervention. In such an event, the cerebrospinal fluid drainage must be suspended(20,21). In the present study, 17% of the G1 population received continuous spinal anesthesia catheter, while in G2, only 10% of the patients received it. Outcomes after endoprosthesis implant in thoracic aortic diseases are favorable both at short and medium terms, with significantly lower rates of early mortality as compared with open surgery. The VALOR(12) trial presents intraoperative mortality rate of 2.1%, while Stone et al. have presented a rate of 10.4% in patients treated for TAA(22). The EURO-STAR registry presents intra-hospital mortality rate of 10%, increasing to 28% in patients submitted to emergency aneurysm repair(8). A protocol for Talent thoracic stent graft for aneurysm repair presented intrahospital mortality in 55 of cases(23). According to Stone et al., the intraoperative mortality reaches 15.1% in this population(22). In the present study, the G1 population presented one death attributed to endovascular intervention and two deaths caused by sepsis resulting from postoperative lower respiratory tract infection. The perioperative mortality rate in this population reaches 10.3%, falling within the variation of values reported in the literature. The Talent thoracic stent graft system clinical study has demonstrated one-year survival rate of 91%. In the present study, G1 presented a survival rate of 89.6%, as no death was recorded within the period of 30 days to one year of follow-up. Differently, the EUROSTAR registry reports a rate of intra-hospital mortality of 8.4% in patients treated for dissection(8). The overall mortality rate of surgical repair for type B dissection is around 30%, reaching 50% in cases where such patients undergo surgery in emergency situations(3). The EUROSTAR registry presented oneyear survival rate of 90% in patients with dissection, while in the series from the Arizona Heart Institute the one-year survival rate was 85%(8,14). The G2 population presented a perioperative mortality rate of 7.6%, with one-year survival of 80.7%. Three deaths occurred after 30 days from intervention: two cases for redissection and one for acute pulmonary edema. The present study demonstrated that the survival rate in G2 is similar to that in other studies, and lower than in G1. It is not clear if endovascular therapy affects the survival of patients who evolve to chronic Stanford type B dissection as compared with clinical treatment. The IN-STEAD trial has compared the medical treatment versus endograft prosthesis in 140 patients with dissection, and no difference was observed in rates of all-cause mortality. The survival rate was 95.6% in the medical treatment group versus 88.9% in the endovascular treatment group, over a two-year follow-up. The rates of progression and death were equal for both groups, while aortic remodeling reached 91.3% in the patients treated by endovascular approach versus 19.4% in the medical treatment group, a fact suggestive of the necessity of endovascular treatment for those patients presenting emerging aortic complications. However, at clinical follow-up, there were a reasonable number of patients who had to migrate to endovascular treatment(24). CONCLUSIONS The experience with thoracic endovascular aortic repair has caused an impact on the clinical practice and is currently becoming the standard treatment both for simple or complex thoracic aorta diseases. The interventions are technically more difficult in the population with true TAAs. In the present study, the endovascular treatment of thoracic aorta diseases has demonstrated to be a feasible method associated with acceptable rates of perioperative complications. The obtained rates of therapeutic success and reintervention demonstrate the need for rigorous and attentive clinical follow-up of such patients. Further studies shall confirm the benefits of endovascular therapy in cases of chronic type B dissection. REFERENCES 1. Bickerstaff LK, Pairolero PC, Hollier LH, et al. Thoracic aortic aneurysms: a population-based study. Surgery. 1982;92:1103-8. 2. Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cadiovasc Surg. 1994;107:1323-33. 3. Trimarchi S, Nienaber CA, Rampoldi V, et al. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2006;114(1 Suppl):I357-64. 4. Palma JH, Buffolo E, Gaia D. Tratamento endovascular das doenças da aorta: visão geral. Rev Bras Cir Cardiovasc. 2009;24(2 Supl 1): 40s-44s. 5. Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546-52. 6. Matalanis G, Durairaj M, Brooks M. A hybrid technique of aortic arch transposition and antegrade stent graft deployment for complete arch repair without cardiopulmonary bypass. Eur J Cardiothorac Surg. 2006;29:611-2. 7. Alves CMR, Fonseca JHP, Souza JAM, et al. Tratamento endovascular nos aneurismas verdadeiros e na dissecção aórtica do tipo B: fase intrahospitalar, seguimento de médio prazo e uma reflexão sobre seleção de pacientes. Rev Bras Cardiol Invas. 2009;17:46-51. 8. Leurs LJ, Bell R, Degrieck Y, et al. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J Vasc Surg. 2004;40:670-80. 9. Chagas Neto FA, Barreto ARF, Reis HF, et al. A importância do diagnóstico por imagem na classificação dos endoleaks como complicação do tratamento endovascular de aneurismas aórticos. Radiol Bras. 2010;43:289-94. 10. Criado FJ, Clark NS, Barnatan MF. Stent-graft repair in the aortic arch and descending thoracic aorta: a 4-year experience. J Vasc Surg. 2002;36:1121-8. 11. Lepore V, Lönn L, Delle M, et al. Endograft therapy for diseases of the descending thoracic aorta: results in 43 high-risk patients. J Endovasc Ther. 2002;9:829-37. 12. Fairman RM, Criado F, Farber M, et al. Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR trial. J Vasc Surg. 2008;48:546-54. 13. Svensson LG, Crawford ES, Hess KR, et al. Variables predictive of outcome in 832 patients undergoing repairs of the descending thoracic aorta. Chest. 1993;104:1248-53. 14. Nathanson DR, Rodriguez-Lopez JA, Ramaiah VG, et al. Endoluminal stent-graft stabilization for thoracic aortic dissection. J Endovasc Ther. 2005;12:354-9. 15. Amabile P, Grisoli D, Giorgi R, et al. Incidence and determinants of spinal cord ischaemia in stent-graft repair of the thoracic aorta. Eur J Vasc Endovasc Surg. 2008;35:455-61. 16. Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II muiticenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41:1-9. 17. Coselli JS, Conklin LD, LeMaire SA. Thoracoab dominal aortic aneurysm repair: review and update of current strategies. Ann Thorac Surg. 2002;74:S1881-4. 18. Palma JH, Souza JAM, Alves CMR, et al. Selfexpandable aortic stent-grafts for treatment of descending aortic dissections. Ann Thorac Surg. 2002;73:1138-42. 19. Elefteriades JA, Hartleroad J, Gusberg RJ, et al. Long-term experience with descending aortic dissection: the complication-specific approach. Ann Thorac Surg. 1992;53:11-21. 20. Cinà CS, Abouzahr L, Arena GO, et al. Cerebrospinal fluid drainage to prevent paraplegia during thoracic and thoracoabominal aortic aneurysm surgery: a systemic review and meta-analysis. J Vasc Surg. 2004;40:36-44. 21. Novero ER, Metzger PB, Angelieri FMR, et al. Correção endovascular do aneurisma da aorta abdominal: análise dos resultados de único centro. Radiol Bras. 2012;45:1-6. 22. Stone DH, Brewster DC, Kwolek CJ, et al. Stentgraft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg. 2006;441188-97. 23. Fattori R, Nienaber CA, Rousseau H, et al. Results of endovascular repair of the thoracic aorta with the Talent Thoracic stent graft: the Talent Thoracic Retrospective Registry. J Thorac Cardiovasc Surg. 2006;132:332-9. 24. Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519-28. 1. Interventional Cardiologists, MDs, Foreign Trainees at Centro de Intervenções Endovasculares (CIEV) - Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 2. Vascular Surgeon, MD, Trainee at Centro de Intervenções Endovasculares (CIEV) - Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 3. Cardiologist, MD, Resident of Vascular Echography, Hospital do Servidor Público Estadual "Francisco Morato de Oliveira" (HSPE), São Paulo, SP, Brazil. 4. PhD, Physician Assistant, Section of Vascular Surgery, Member of Centro de Intervenções Endovasculares (CIEV) - Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 5. Vascular Surgeon, Physician Assistant, Section of Radiology, Member of Centro de Intervenções Endovasculares (CIEV) - Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 6. PhD, Head of Sector of Vascular Surgery and Centro de Intervenções Endovasculares (CIEV) - Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 7. PhD, Head of Section of Radiology and Centro de Intervenções Endovasculares (CIEV) - Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. Mailing Address: Dr. Patrick Bastos Metzger Instituto Dante Pazzanese de Cardiologia Rua Doutor Dante Pazzanese, 500, Vila Mariana São Paulo, SP, Brazil, 04012-180 E-mail: patrickvascular@gmail.com Received April 6, 2012. Accepted after revision August 9, 2012. * Study developed at Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554