INTRODUCTION
Non-small cell lung cancer (NSCLC) corresponds to approximately 85% of all lung neoplasms, and is the main cause of deaths from lung cancer in Brazil nowadays. According to Instituto Nacional de Câncer (INCA) (National Cancer Institute), the estimated mortality due to NSCLC in Brazil reached 14,715 patients in the year of 2000(1), with 27,270 new cases in 2008(2).
The initial staging of NSCLC determines the best treatment approach and is essential to define the patient's prognosis. Thus, an incorrect staging of NSCLC may lead to inappropriate treatment (futile surgeries in patients with advanced disease, as well as contraindication for curative surgery in patients with an otherwise curable disease)(5,6).
The TNM classification of malignant tumors, developed by the American Joint Committee on Cancer, is the most commonly utilized staging system, and is based on tumor size, regional lymph nodes involvement and presence of metastasis. The definition of the nodal stage (N) is particularly important in the decision making regarding neoadjuvant therapy to be adopted before surgical resection, potentially increasing the long-term survival of patients with stage IIIA NSCLC(7-9). Several diagnostic tools have been investigated for the early detection and staging of NSCLC, including chest radiography, computed tomography (CT), magnetic resonance imaging (MRI), bronchoscopy, videothoracoscopy, transesophageal ultrasonography (EUS) or transbronchial ultrasonography (EBUS) and mediastinoscopy.
Positron emission tomography (PET) with [
18F]-fluoro-2-deoxy-D-glucose (FDG) has consistently played an important role in the noninvasive preoperative staging of patients with NSCLC(10-12). As compared with CT, FDG-PET is more accurate in N and M staging and, therefore, has a considerable clinical impact in the identification of unresectable disease(13-15). However, the additional costs arising from the introduction of this new technology must be determined. Thus, the present study is aimed at assessing the impact of metabolic staging (MS) with FDG-PET in the initial staging of patients with NSCLC in Brazil and determine the cost-effectiveness of such a strategy as compared with the conventional staging (CS).
MATERIALS AND METHODS
Patients
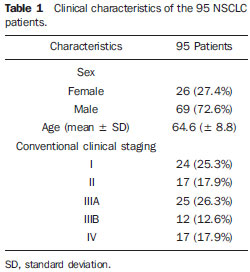
The present prospective study, approved by the Committee for Ethics in Research of Universidade de São Paulo/Hospital das Clínicas, included 95 consecutive patients with recent NSCLC diagnosis confirmed by biopsy. The patients were referred by the Service of Pneumology of Instituto do Coração (InCor) - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP). A term of free and informed consent was obtained from all eligible patients in the period between August 2005 and December 2007. Pregnancy and presence of other concomitant tumor were the exclusion criteria.
All the 95 patients were submitted to CS and MS, while MRI and scintigraphy were only performed in cases of clinical relevance.
Conventional staging
All the patients underwent CS, including physical examination, laboratory tests (LDH, alkaline phosphatase, liver enzymes, bilirubin, renal function, calcium) and CT (chest, abdomen and pelvis). At CT, slice thickness was 1 mm thickness, with administration of both oral and intravenous contrast.
Cranial MRI and bone scintigraphy were performed only in the presence of neurological symptoms and bone pain, respectively. EUS with biopsy was performed in the suspicion of mediastinal lymph nodes involvement.
The conventional clinical stage for each patient was attributed in accordance with "The revised TNM staging for lung cancer"(16).
FDG-PET
Tomographic images were acquired from the skull to the roots of the thighs in a dedicated PET apparatus (GE Advance; GE Healthcare, Waukesha, WI, USA), 60 to 90 minutes following intravenous injection of 296-444 MBq (8-12 mCi) of FDG. The images were reconstructed in the axial, sagittal and coronal planes. Attenuation correction was performed with
68Ge sources.
The images were interpreted by two experienced nuclear physicians, both bearing nuclear medicine specialist title. The FDG-PET images were analyzed together with the CT images. Areas of non-physiological concentration with FDG concentration greater than the background concentration were classified as positive for disease. The metabolic stage for each patient was attributed according to the TNM staging system.
The time elapsed between the performance of CT and PET was not longer than two weeks.
Treatment and follow-up
The final TNM staging was obtained at a consensus meeting by a group of oncologists and imaging diagnosis specialists on the basis of all available data (clinical data, initial CT and PET-FDG, bronchoscopy, mediastinoscopy and EUS, as applicable). EUS was considered the reference standard in the preoperative evaluation of mediastinal lymph nodes.
For treatment planning purposes, the patients were divided into groups as follows: (i) stage I: curative surgery; (ii) stages II and IIIA (resectable disease): curative surgery with adjuvant chemotherapy; (iii) stage IIIB: combined chemotherapy-radiotherapy; (iv) stage IV: palliative chemotherapy. Treatment changes after MS were determined in cases of changes in the original treatment plan with basis on FDG-PET results. Positive findings only at FDG-PET study were confirmed by biopsy or TEUS at the discretion of the assisting physician.
All the patients were followed-up according to the local conventional standard, being evaluated at every three months or at shorter intervals, for at least one year.
Reference standard and data analysis
Considering that the histological analysis of all possible sites of involvement by NSCLC is not feasible for obvious ethical and practical reasons, the definition of the diagnostic methods accuracy was based on combined results from conventional methods and FDG-PET to define a reference standard. Intermethod agreement on positive findings at CT and PET (or at bone scintigraphy and MRI, if applicable) has led to interpretation of such findings as true-positive. Agreement between negative clinical and imaging findings has led to interpretation of such findings as true-negative. In cases of disagreeing nodal stages (N) at MS and CS, the criteria utilized to define the result was the anatomopathological evaluation by means of EUS at stages IIIA or IIIB, or by lymph nodes sampling at thoracotomy, or development of local recurrence, distant metastasis or death up to one year after thoracotomy (stage IV of the disease). The data were prospectively collected.
In the clinical stage analysis, CS and MS results were compared in cases where FDG-PET indicated a change in the surgical approach as the patient stage changed from I-IIIA to IIIB-IV, and vice-versa; and in radiotherapy, as the stage changed from IIIB to IV and vice-versa.
Regarding patients considered eligible for surgery at CS and MS, thoracotomy was considered as futile in cases where anatomopathological evaluation of the surgical specimens confirmed disease at stage IIIB or IV, or in the case of recurrence or death due to any cause up to one year after surgery.
The diagnostic accuracy was analyzed by summing up the number of patients with true-positive and true-negative results, and dividing the result by the total number of patients. The intermethod agreement was evaluated by means of the McNemar χ
2 test. The software SPSS 10.0 for Windows (SPSS Inc.; Chicago, IL, USA) was utilized for statistical analysis.
Cost-effectiveness analysis
All the health care resources required for patients' assessment and treatment were prospectively evaluated and quantified. Drugs, procedures (including investigations, chemotherapy, radiotherapy, surgical procedures, days of hospitalization, outpatient and hospital assistance) or any other resources utilized for local care were calculated by micro-costing methodology. The HC-FMUSP is a public hospital, and all material purchase is performed by means of electronic tendering in statewide bids. The costs of resources were updated for 2010. Medical fees were not included.
The clinical stage of the patient defines which treatment he/she should undergo. The mean cost of each staging of the disease was calculated for each group. The economic impact of CS (strategy I), CS + FDG-PET (strategy II), and PET/CT (strategy III) was calculated.
The calculations of costs for staging and first-line treatment strategies were based on the actual costs of strategy II (CS + FDG-PET) and estimated for strategies I and III.
RESULTS
The patients' clinical characteristics are described on Table 1.
Accuracy and impact on changes in CS and in therapy
Table 2 shows the comparison of CS and MS with FDG-PET. There was agreement between CS and MS in 44/95 (46.3%) of the patients. Intermethod agreement was not observed in 51/95 patients (53.7%): 46/95 (48.4%) patients had their staging changed to more advanced stages with the information provided by FDG-PET (Figure 1), while 5/95 (5.3%) patients had their staging modified to earlier stages (Figure 2).
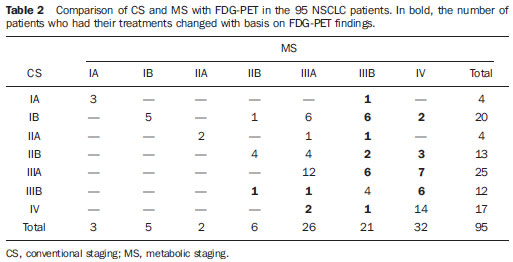
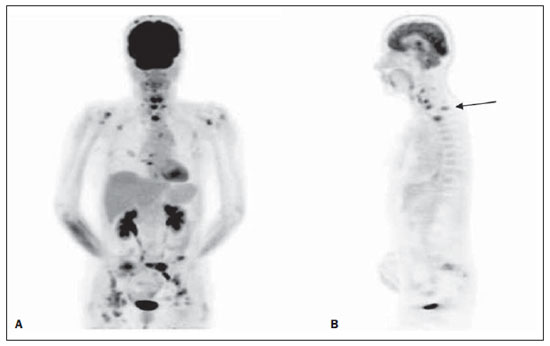
Figure 1. Female patient, smoker, at initial NSCLC staging. At CS, patient presented operable disease. However, FDG-PET scan demonstrated increased FDG uptake bilaterally in cervical lymph nodes and in the mediastinum, besides bone metastasis (A: 3D reformation), notably in the cervical and thoracic spine (B: sagittal view).
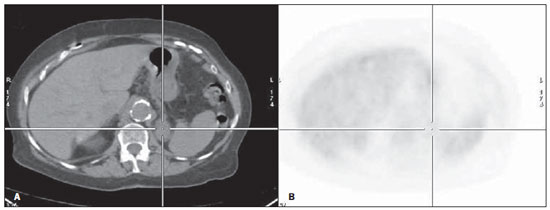
Figure 2. Male patient, smoker, at initial NSCLC staging. At CS, patient presented inoperable disease. Probable left adrenal metastasis at CT (A: axial section), however without increase in metabolism (B: axial section). Follow-up studies did not demonstrated any change in the the left adrenal gland.
Considering all the cases in the present study, the inclusion of FDG-PET in CS would change the treatment in 39/95 patients (41.0%) as regards the surgical therapeutic approach (I-IIIA ↔ IIIB-IV), and in 7/95 patients (7.4%) (stage IIIB ↔ IV) as regards radiotherapy.
As regards the reference standard, PET has correctly staged 86/95 patients (90.5%) as compared with 53/95 cases (55.8%) with CS. Results of PET were considered as false-positive in 8 patients, and CT, in four cases; while PET results were considered as false-negative in one patient, and CT in 38 patients. Statistically significant difference was observed between CT and PET results (MacNemar test χ
2:
p < 0.001).
As regards the final treatment, information from PET would determine change in treatment for 39 patients; in 9 cases the treatment was that suggested by CS, while in 30 patients the results were defined by PET, avoiding surgery in 21 patients, suggesting surgery for two patients and radiotherapy for six patients, and avoiding it for one patient. Finally, considering the final treatment result, MS has correctly defined the treatment in 86/95 (90.5%) of the patients, and CS in 65/95 (68.4%).
Futile thoracotomy
The staging data determined the indication for curative surgical treatment with thoracotomy in 33 patients submitted to MS and in 66 patients submitted to CS.
Among the 66 patients with indication for surgical treatment by CS, the procedure was considered futile in 31 patients (47%): 11 patients with confirmed stage IV, 9 were reclassified as inoperable NSCLC based on anatomopathological findings by EUS, and 15 died less than one year after thoracotomy.
Considering MS, 8/42 patients (19%) would be submitted to futile thoracotomy: one inoperable case based on EUS, one patient with stage IV, who died, besides six other patients (four patients stage 3A and two stage 2B) who also died less than one year after thoracotomy.
Local costs analysis
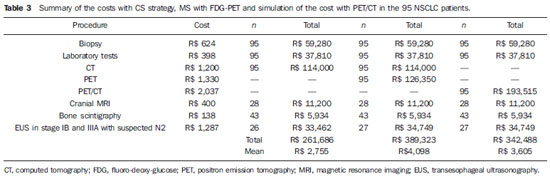
The mean costs of the procedures were the following: biopsy, R$ 624; CT, R$ 1,200; MRI, R$ 400; laboratory and biochemistry, R$ 398; EUS, R$ 1,287; FDG-PET, R$ 1,330; PET/CT, R$ 2,037.The costs of the three different strategies are presented on Table 3.
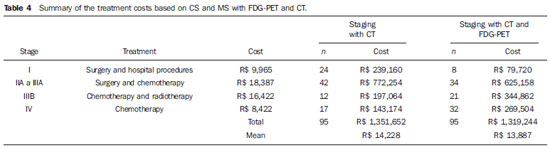
The mean costs of each treatment modality were the following: surgery and hospital procedures, R$ 9,965; adjuvant chemotherapy, R$ 8,422; radiotherapy, R$ 8,000. The costs of staging and first-line treatment are presented on Table 4.
The initial staging of the 95 patients with CT had a total cost of R$ 114,000, while that cost with FDG-PET was R$ 126,350. The calculated cost for PET/CT would be R$ 193,515 for the same patients. The MS by means of FDG-PET would change the disease stage in a considerable number of patients, determining changes in treatment strategies in 39/95 patients (41.0%).
The number of supplementary studies, such as cranial MRI and bone scintigraphy would remain the same with both strategies. However, in CS, EUS would be performed in 26 patients with stages IB to IIIA with N2 disease; and in MS with strategy II or III the EUS would be performed in 27 patients.
The cost of futile thoracotomy in 31 conventionally staged patients reached R$ 308,915, while in eight patients with MS, the cost reached R$ 79,720, representing total savings of R$ 229,195.
The mean cost of staging and first-line treatment with strategy I by CS would be R$ 16,983 per patient; with strategy II (CT + PET) it would be R$ 17,985; and with strategy III (PET/CT) the mean cost would be R$ 17,492 per patient.
DISCUSSION
Patients with NSCL at clinical stages IA, IB, IIA, IIB of the disease might benefit from curative surgical resection. Patients with stages IIIB and IV are inoperable, while patients with stage IIIA rarely meet the criteria for surgery. The current role of neoadjuvant chemotherapy for selected patients with stage IIIA remains controversial(16).
The present study results confirm the benefits of MS as compared with CS, leading to a more accurate definition of the disease stage and, therefore, an increasingly more refined treatment strategy for the patient. The data from FDG-PET resulted in the modification of the stage in approximately half the cases (51/95; 53.7%) and changes in treatment in approximately onethird (30/95; 31.6%) of the cases, particularly avoiding unnecessary treatment in one-third of the patients (21 cases had contraindications for surgery, and 6 cases for radiotherapy) while suggesting more aggressive treatment for only three patients (surgical treatment for two patients and radiotherapy for one patient).
Several other studies have demonstrated the significant clinical impact of FDG-PET on the initial staging of NSCLC patients, ranging from 22% to 67%(5,17-19). As CS and CS + MS strategies are compared, it is also important to consider the costs of exams and subsequent procedures performed for the investigation of the results of such new method. In the present study, the investigation strategy including FDG-PET increased the number of subsequent procedures in only one of the 95 patients.
Randomized studies(5,20,21) suggest that FDG-PET and FDG-PET/CT improve the accuracy in the mediastinal staging of NSCLC, as compared with CT, confirming the results reported by previous non-randomized studies(22-28). Typically, hybrid PET/CT devices present a better performance than PET alone, optimizing the interpretation of both modalities as it allows for the anatomical identification of areas with increased metabolism(29-33).
Three important randomized studies reported the impact of adding FDG-PET to conventional staging for NSCLC(5,21,22). Their results were different. Viney et al.(20) have faced as primary result the performance of thoracotomy, which was performed in 98% of their patients of the CS group and in 96% of the group MS with FDG-PET besides CS (
p = 0.44), and demonstrated that the addition of PET did not significantly reduce the number of thoracotomies. The main result of the study developed by van Tinteren et al.(5) was futile thoracotomy. The rate of futile thoracotomies was 41% in the CS group and 21% in the group of MS with PET in association with CS (
p < 0.003). In 2009, Fischer et al.(21) randomized the investigation by means of CS and MS followed by the in-vestigation of any abnormality. The rate of futile thoracotomies was 52% in the CS group and 35% in the group with CS associated with MS. The present study results are in agreement with those of the latter two studies, demonstrating the benefits of MS, with a significantly lower rate of futile thoracotomies in comparison with the rate obtained by CS (19% versus 47%).
There are scarce studies approaching the utilization of FDG-PET in Brazilian patients(34,35), and studies approaching analysis of its cost-effectiveness are even rarer(36). The rational utilization of financial resources is extremely relevant for the public health system, moreover in developing countries such as Brazil. Additionally, one should take into consideration that results of cost-effectiveness studies in certain countries may not necessarily apply to others. In the case of the present study, the results corroborate those of studies developed in other countries and demonstrate the cost-effectiveness of MS with FDG-PET in cases of NSCLC. The costs of futile thoracotomies in eight patients with MS reached R$ 79.720, while those costs in 31 patients with CS reached R$ 308.915. Just such saving in costs (R$ 229.195) would be more than enough to cover the costs of all FDG-PETs (R$ 126,350) or even FDG-PET/CTs (R$ 193,515) for all of the 95 patients.
One should take into consideration that the present study results may be questioned with respect to the utilization of PET apparatuses instead of PET/CT apparatuses for staging of NSCLC. However, it is interesting to notice that the data regarding accuracy data in the present study are quite similar to reported data for PET/CT. This can be explained by the careful correlation of the PET and CT images during the reading of the FDG-PET studies. The main disadvantage in the present study is the absence of histological confirmation of all the lesions. Until recently, mediastinoscopy was considered as being the gold standard for N staging(16). However, new and less invasive technologies are emerging, such as the case of EUS and EBUS. Thus, due to superimposition of anatomical areas, mediastinoscopy was not mandatory in the present study. Additionally, a randomized and controlled study would have been more elegant, however, ethical considerations impaired such option.
CONCLUSION
The present study demonstrates that MS with FDG-PET is more accurate than CS with CT. Additionally, FDG-PET is a costeffective imaging method in initial staging of NSCLC, allowing a better selection of patients for the most appropriate treatment in approximately one-half of the cases and reducing the number of futile thoracotomies from 47% to 19%.
The introduction of MS with FDG-PET or FDG-PET/CT is a very cost-effective option, with acceptable cost for NSCLC staging, and therefore, its utilization is economically justifiable in the Brazilian public health system.
REFERENCES
1. Instituto Nacional de Câncer. Tipos de câncer - pulmão. [acessado em 3 de julho de 2012]. Disponível em: http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/pulmao
2. Instituto Nacional de Câncer. Incidência de câncer no Brasil - estimativa 2007. [acessado em 4 de fevereiro de 2011]. Disponível em: http://www.inca.gov.br/estimativa/2007
3. Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584-94.
4. Tanoue LT. Staging of non-small cell lung cancer. Semin Respir Crit Care Med. 2008;29:248-60.
5. van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multi-centre randomised trial. Lancet. 2002;359: 1388-93.
6. Swensen SJ, Brown LR, Colby TV, et al. Lung nodule enhancement at CT: prospective findings. Radiology. 1996;201:447-55.
7. Rosell R, Felip E. Role of multimodality treatment for lung cancer. Semin Surg Oncol. 2000;18:143-51.
8. Spásová I, Petera J, Hytych V. The role of neoadjuvant chemotherapy in marginally resectable or unresectable stage III non-small cell lung cancer. Neoplasma. 2002;49:189-96.
9. Roth JA, Atkinson EN, Fossella F, et al. Longterm follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1-6.
10. Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123(1 Suppl):137S-146S.
11. Vesselle H, Pugsley JM, Vallières E, et al. The impact of fluorodeoxyglucose F 18 positron-emission tomography on the surgical staging of nonsmall cell lung cancer. J Thorac Cardiovasc Surg. 2002;124:511-9.
12. Graeter TP, Hellwig D, Hoffmann K, et al. Mediastinal lymph node staging in suspected lung cancer: comparison of positron emission tomography with F-18-fluorodeoxyglucose and mediastinoscopy. Ann Thorac Surg. 2003;75:231-6.
13. Kalff V, Hicks RJ, MacManus MP, et al. Clinical impact of (18)F fluorodeoxyglucose positron emission tomography in patients with non-smallcell lung cancer: a prospective study. J Clin Oncol. 2001;19:111-8.
14. Lowe VJ, Naunheim KS. Positron emission tomography in lung cancer. Ann Thorac Surg. 1998;65:1821-9.
15. Stroobants S, Verschakelen J, Vansteenkiste J. Value of FDG-PET in the management of nonsmall cell lung cancer. Eur J Radiol. 2003;45:49-59.
16. Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):178S-201S.
17. Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254-61.
18. Miele E, Spinelli GP, Tomao F, et al. Positron emission tomography (PET) radiotracers in oncology - utility of 18F-fluoro-deoxy-glucose (FDG)-PET in the management of patients with non-small-cell lung cancer (NSCLC). J Exp Clin Cancer Res. 2008;27:52.
19. Subedi N, Scarsbrook A, Darby M, et al. The clinical impact of integrated FDG PET-CT on management decisions in patients with lung cancer. Lung Cancer. 2009;64:301-7.
20. Viney RC, Boyer MJ, King MT, et al. Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol. 2004;22:2357-62.
21. Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361:32-9. Erratum in: N Engl J Med. 2011;364:982.
22. Herder GJ, Kramer H, Hoekstra OS, et al. Traditional versus up-front [18F] fluorodeoxyglucosepositron emission tomography staging of nonsmall-cell lung cancer: a Dutch cooperative randomized study. J Clin Oncol. 2006;24:1800-6.
23. [No authors listed]. Investigating extrathoracic metastatic disease in patients with apparently operable lung cancer. The Canadian Lung Oncology Group. Ann Thorac Surg. 2001;71:425-34.
24. Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254-61.
25. Saunders CA, Dussek JE, O'Doherty MJ, et al. Evaluation of fluorine-18-fluorodeoxyglucose whole body positron emission tomography imaging in the staging of lung cancer. Ann Thorac Surg. 1999;67:790-7.
26. Weder W, Schmid RA, Bruchhaus H, et al. Detection of extrathoracic metastases by positron emission tomography in lung cancer. Ann Thorac Surg. 1998;66:886-93.
27. MacManus MP, Hicks RJ, Ball DL, et al. F-18 fluorodeoxyglucose positron emission tomography staging in radical radiotherapy candidates with nonsmall cell lung carcinoma: powerful correlation with survival and high impact on treatment. Cancer. 2001;92:886-95.
28. Ung YC, Maziak DE, Vanderveen JA, et al; Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care. 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst. 2007;99:1753-67.
29. Hicks RJ, Kalff V, MacManus MP, et al. (18)F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med. 2001;42:1596-604.
30. Cerfolio RJ, Ojha B, Bryant AS, et al. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004;78:1017-23.
31. Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500-7.
32. Halpern BS, Schiepers C, Weber WA, et al. Presurgical staging of non-small cell lung cancer: positron emission tomography, integrated positron emission tomography/CT, and software image fusion. Chest. 2005;128:2289-97.
33. Antoch G, Stattaus J, Nemat AT, et al. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology. 2003;229:526-33.
34. Cerci JJ, Pracchia LF, Linardi CC, et al. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med. 2010;51:1337-43. Erratum in: J Nucl Med. 2010;51:1658.
35. Pracchia LF, Chaves AA, Cerci JJ, et al. Metabolic test with fluorine-18-fluorodeoxyglucose in staging and detection of residual tumor or recurrence in Hodgkin lymphoma. Clinics (Sao Paulo). 2007;62:121-6.
36. Cerci JJ, Trindade E, Pracchia LF, et al. Cost effectiveness of positron emission tomography in patients with Hodgkin's lymphoma in unconfirmed complete remission or partial remission after first-line therapy. J Clin Oncol. 2010;28:1415-21.
1. PhD, Director of PET-CT Center, Quanta - Diagnóstico e Terapia, Curitiba, PR, MD, Researcher, Instituto do Coração (In-Cor) - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil.
2. Postdoctoral Fellowship, Professor, Universidade de São Paulo (USP), São Paulo, SP, Brazil.
3. PhD, Núcleo de Avaliação de Tecnologia, Instituto do Coração (InCor) - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil.
4. Nuclear Physician, Researcher, Unit of Nuclear Medicine, Instituto do Coração (InCor) - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil.
5. MDs, Pneumologists, Unit of Pneumology, Instituto do Coração (InCor) - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil.
6. PhD, Director of PET-CT Center, Unit of Nuclear Medicine, Instituto do Coração (InCor) - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil.
7. PhD, Director of Unit of Pneumology, Instituto do Coração (InCor) - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil.
Mailing Address:
Dr. Juliano Julio Cerci
Núcleo de Avaliação de Tecnologia do Instituto do Coração (InCor) - FMUSP
Avenida Doutor Enéas de Carvalho Aguiar, 44, AB, Cerqueira César
São Paulo, SP, Brazil, 05403-000
E-mail: cercijuliano@ hotmail.com
Received May 15, 2012.
Accepted after revision June 28, 2012.
Study developed at Instituto do Coração (InCor) - Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil. The present study was sponsored by the Brazilian Ministry of Health.
 Vol. 45 nº 4 - July / Aug. of 2012
Vol. 45 nº 4 - July / Aug. of 2012