INTRODUCTION
Breast cancer is the most frequent type of cancer and is most prevalent in women, accounting for about 22% of new cases of cancer annually (1). Despite the high incidence of this disease, the breast cancer mortality rate has been decreasing due to early detection and to the currently available imaging technology(2).
Because of its poor sensitivity and specificity, multislice computed tomography (MSCT) does not present considerable evidences of a positive cost-benefit ratio in the evaluation of the breast parenchyma( 3), but its significant role in the staging and follow-up of breast cancer patients should be highlighted considering its usefulness in the determination of the therapy to be adopted as well as of the patient's prognosis.
A consensus is still to be achieved on a formal indication for MSCT in the absence os symptoms or clinical indications which justify the request of such study(4), considering the higher sensitivity of positron emission computed tomography(5).
The present study was aimed at demonstrating MSCT findings of extramammary changes detected during breast cancer staging and follow-up of patients submitted to treatment.
POSTOPERATIVE APPEARANCE OF THE CHEST WALL
The postoperative appearance of the chest wall varies with the adopted surgical technique as follows: 1) radical mastectomy (Figure 1A) implies removal of breast, pectoralis major and minor muscles and regional lymph nodes along the axillary vein up to the costoclavicular ligament; 2) modified radical mastectomy – an alternative method to conservative treatment – involves mastectomy and dissection of axillary lymph nodes, sparing the pectoralis major muscle (Patey's mastectomy) (Figure 1B), or sparing both pectoralis major and minor muscles (Auchincloss' procedure)(6).
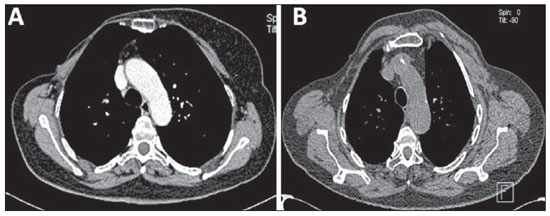
Figure 1. Postoperative appearance of chest wall at MSCT.
A: Axial image of total radical mastectomy demonstrating chest wall asymmetry, and absence of both pectoralis major and minor muscles at right.
B: Axial image of modified radical mastectomy (Patey's mastectomy) where the absence of the pectoralis minor muscle is observed at right.
Other more conservative reconstruction methods may be adopted. The most common surgical complication is the occurrence of seroma, besides infections, necrosis, lymphedema and axillary contracture( 6).
POST-RADIOTHERAPY CHEST MANIFESTATIONS
Radiotherapy has been widely utilized following surgery in breast cancer patients to reduce the risk for locoregional recurrence and/or increase in tumor volume in cases of advanced disease(7).
Frequently, radiotherapy causes radiation pneumonitis, occurring about 4 and 12 weeks after treatment completion, and generally remaining confined to the irradiation region. Initially, the disease manifests with linear opacities or consolidation. Such changes may either gradually disappear or result in signs of evolutive fibrotic changes along a period between six months and two years, tending to remain stable after a two-year evolutive period(6,8) (Figure 2).
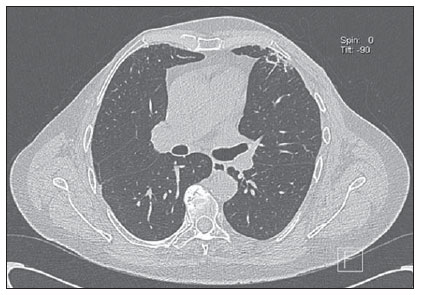
Figure 2. Post-radiotherapy chest manifestation. Axial MSCT image demonstrating actinic lesion in left lung of a patient submitted to radiotherapy for breast cancer.
Three irradiation fields induce radiation pneumonitis in breast cancer patients, namely, tangential field, supraclavicular field and internal mammary field. The tangential field utilized to irradiate the chest wall results in pneumonitis in the peripheral lung anterolaterally, with a typical shape. The supraclavicular field may induce changes in the apex of the lung, resulting in lesions similar to those observed in pulmonary tuberculosis. The internal mammary field, utilized to irradiate internal mammary lymph nodes, may induce changes in the paramediastinal region. In cases where areas of opacity are seen at post-radiotherapy follow-up radiography, the differential diagnoses include radiation pneumonitis, local recurrence, lymphangitic spread and infectious pneumonitis( 6).
POST-CHEMOTHERAPY MANIFESTATIONS
The main chemotherapy agents utilized in the treatment of breast cancer are cyclophosphamide, mathotrexate, 5-fluorouracil and doxorubicin, and the major complications to be taken into consideration include pneumonitis, cardiotoxicity and infections.
Chemotherapy-induced cardiotoxicity caused by doxorubicin is generally dosedependent and is initially asymptomatic, progressing to transient arrhythmia up to fatal cardiomyopathy caused by permanent left ventricular dysfunction. At MSCT, such condition is seen as cardiomegaly and/or pericardial effusion(9) (Figure 3).
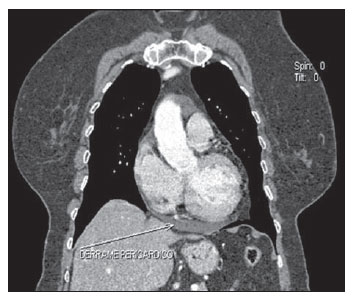
Figure 3. Post-chemotherapy manifestation. Coronal MSCT image demonstrating pericardial effusion in patients under chemotherapy for breast cancer.
Interstitial lung diseases refer to a wide and heterogeneous group of fibrotic pulmonary diseases, including interstitial pneumonitis. In most of cases, the cause of interstitial pneumonitis is unknown. Lung toxicity induced by pharmaceuticals indicates a possibly subdiagnosed etiology of interstitial lung diseases. It has been demonstrated that currently preconized treatments for breast cancer including tamoxifen and taxanes increase the risk for interstitial pneumonitis, particularly in cases of combination with adjuvant radiotherapy. The most frequent pulmonary findings resulting from chemotherapy drugs toxicity include interstitial pattern, groundglass opacity and consolidation (Figure 4), the latter occasionally simulating a nodule or mass(10).
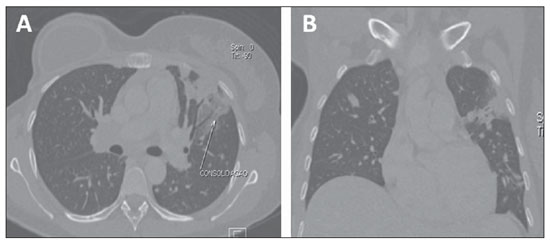
Figure 4. Post-chemotherapy manifestation. Axial (
A) and coronal (
B) MSCT demonstrating pulmonary consolidation resulting from chemotherapy in a breast cancer patient.
Local recurrence is defined as reappearance of a tumor in the surgical site, occurring in 34–84% of cases; and regional recurrence is characterized by the appearance of metastasis in lymph nodes involved in lymphatic drainage, including supraclavicular, axillary lymph nodes and those of the internal mammary chain(11).
The detection of recurrent breast cancer by mammography represents a challenge due to architectural changes, fibrosis and parenchymal scarring secondary to surgery and radiotherapy which make the interpretation of the images more difficult(12).
Generally, patients with involvement of four or more axillary lymph nodes present a significant risk for recurrence which, at MSCT, is seen as multiple lymph nodes with increased diameter. Frequently, metastases to lymph nodes of the internal mammary chain (Figure 5) and mediastinum are clinically occult because of their size, producing equivocal findings at MSCT(13).
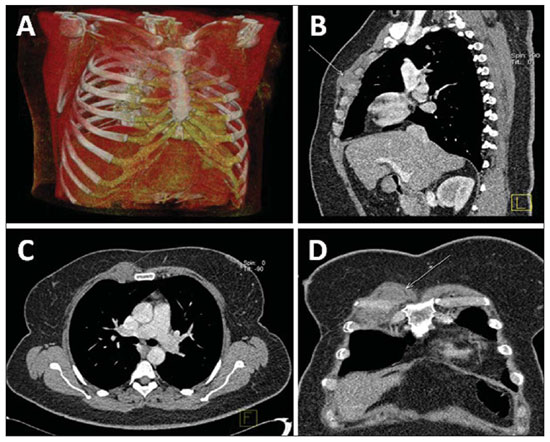
Figure 5. Lymph node enlargement in right internal mammary chain. Regional recurrence six months after conservative surgery. Chest MSCT with volume rendering (
A), sagittal (
B), axial (
C) and coronal (
D) images.
Tomographic findings suggestive of local recurrence include focal thickening > 1 cm, presence of nodular lesions in the subcutaneous tissue or in muscles of the chest wall, and contours irregularity and pectoralis muscles heterogeneity(11).
Normal lymph nodes of the internal mammary chain are less than 5 mm in diameter and metastases to this chain cannot be easily detected at clinical examination, mammography or ultrasonography, since they are covered by bony and cartilaginous structures of the chest wall. Normal lymph nodes cannot be routinely identified by computed tomography. Therefore, detectable lymph nodes of the internal mammary chain with > 6 mm in diameter in breast cancer patients suggest the presence of malignant lymphadenopathy(14).
Disease recurrence in the internal mammary chain is rarely observed, occurring with a wide variation in 8–37% of cases, but is concomitantly present in 44% of cases with axillary involvement(11).
LYMPH NODE COMPROMISE
Lymph node metastasis (Figures 6 and 7) is relatively frequent in breast cancer, and an appropriate staging of the tumor is relevant to optimize the diagnostic workup(15).
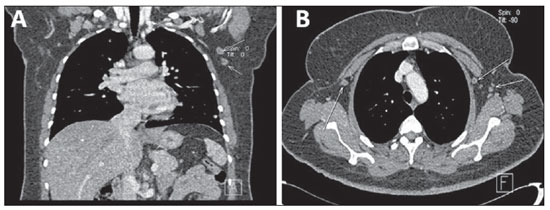
Figure 6. Lymph node compromise. Coronal (
A) an axial (
B) MSCT images demonstrating axillary lymph nodes bilaterally, some of them with increased diameter.
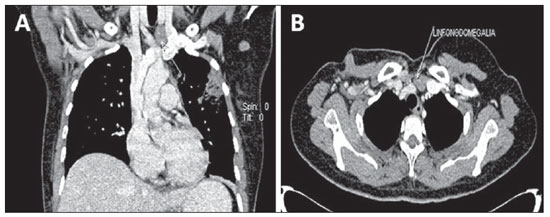
Figure 7. Lymph node compromise. Coronal (
A) and axial (
B) MSCT images demonstrating supraclavicular and axillary lymph nodes enlargement at left.
Despite its proved capacity to reduce morbidity, sentinel lymph node biopsy is an invasive procedure(16) and, therefore, the acquisition of lymph node images by contrast- enhanced MSCT seems to be more convenient, despite being an indirect diagnostic method(17).
Computed tomography is the imaging method of choice for detecting lymph node compromise, many times defining the disease staging according to the TNM classification, in spite of not having value in the evaluation of tumor size. Additionally, the performance of this method in cancer detection and quantification is significantly reduced in cases where the lesion diameter is < 1 cm(13,18,19).
PLEURAL AND PULMONARY METASTASIS
The pleura if a frequent target of metastasis in breast cancer patients (Figure 8), and pleural effusion ipsilateral to the primary tumor is the most common sign of such metastasis, probably by lymphatic dissemination. Breast cancer metastasis is one of the three major causes for malignant effusion( 20).
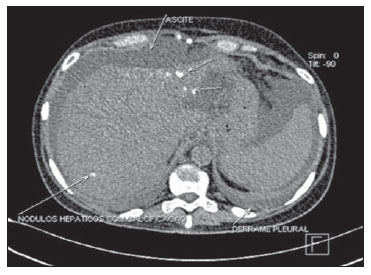
Figure 8. Pleural metastasis. Axial MSCT image showing pleural metastasis in a breast cancer patient. Note the presence of calcified liver metastases (patient previously submitted to chemotherapy) and ascites.
Nodularity, irregular thickening and pleural plaque constitute less common findings in pleural metastasis, and rarely occur without association with pleural effusion(6).
Multiple nodules occurring by hematogenic tumor dissemination (Figure 9) are common findings in cases of pulmonary metastasis from breast cancer. Generally, metastatic lesions present a spherical/ovoid shape, with variable sizes, well defined margins, and most of times located peripherally to the lungs, sometimes found as calcified nodules(6,20,21).
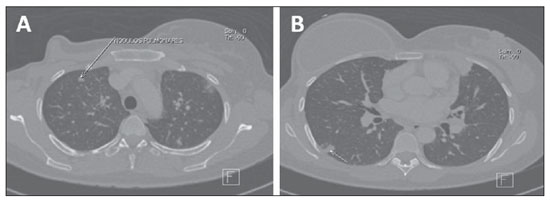
Figure 9. Lung metastasis. Axial MSCT images demonstrating metastatic pulmonary nodules in a patient submitted to right total mastectomy for breast cancer.
The detection of a solitary lung nodule in patients previously treated for breast cancer does not necessarily represent metastatic disease. In many cases, a solitary lung nodule is originated from a primary pulmonary carcinoma. However, in patients with extrathoracic malignancy, the chance for metastasis corresponds to 25%(6,21).
Other manifestations of pulmonary metastasis are carcinomatous lymphangitis and centrolobular nodules, both resulting from endobronchial dissemination(6).
BONE MESTASTASIS
Bone metastasis is the second most common type of breast cancer distant metastasis, causing high morbidity because of pain, mobility compromise, hypercalcemia, pathological fracture, compression of the dural sac, spinal cord or nerve roots, and bone marrow infiltration. Breast cancer is the most common cause for medullary compression in women(6).
Several imaging methods are available for evaluating bone metastasis in breast cancer patients. Bone scintigraphy is suggested as a first imaging technique for asymptomatic patients, considering the high sensitivity of this method in the detection of bone metastasis, allowing excellent skeletal evaluation(22).
Radiologically, the majority of bone metastases are multiple and may be osteolytic, osteoblastic or a combination of both types(6, 23,24) (Figure 10). It is important to highlight that after radiotherapy, chemotherapy or hormone-based therapy, initially osteolytic metastases may become osteosclerotic.
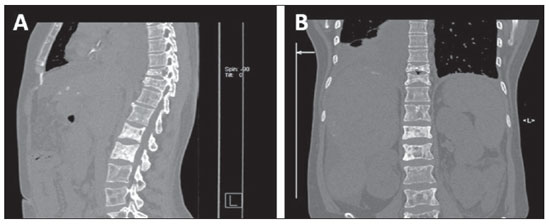
Figure 10. Bone metastasis. Sagittal (
A) and coronal (
B) MSCT images demonstrating mixed type bone metastases (osteolytic and osteoblastic metastases) in the dorsal and lumbar spine of a breast cancer patient presenting dorsolumbar pain.
Approximately 50% of women with metastatic breast cancer present liver metastasis in the course of the disease. Radiographically, liver metastases present several appearances as follows: "target" lesions at ultrasonography, and hypoattenuating at portal phase computed tomography, because of their hypovascularization(25,26) (Figure 11). Generally, they are non-calcified nodular lesions, and may present calcifications after chemotherapy treatment (Figure 8).
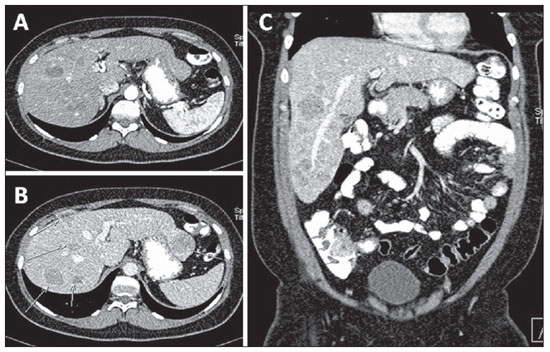
Figure 11. Liver metastasis. Axial, arterial phase (
A) portal phase (
B) and coronal, portal phase (
C), images demonstrating hypovascularized liver metastasis in a breast cancer patient.
Studies have demonstrated that liver metastases can be identified as hypervascularized at contrast-enhanced arterialphase CT. Hypervascularized lesions are less common and may appear isoattenuating during the portal phase(26,27).
The typical enhancement pattern of breast cancer metastases corresponds to a subtle peripheral enhancement during the arterial phase, and a more intense enhancement during the portal phase. Other typical pattern of contrast-enhancement of breast cancer metastasis is that of a lesion with minimal or no peripheral enhancement after intravenous contrast injection in the arterial phase and no enhancement in the portal phase, becoming noticeable in this phase for presenting hypoattenuation in relation to the hepatic parenchyma.
BRAIN METASTASIS
Breast cancer is responsible for approximately 10–15% of cases of brain metastasis (Figure 12), which in 70–80% of cases present as multiple lesions many times diagnosed after alterations in other systemic alterations. The most frequent locations of brain metastases are the grey-white matter junction and vascular borders followed by deep parenchymal structures and posteriorly in the cerebral trunk, with supratentorial regions being more frequently affected than infratentorial regions. Brain metastases may also occur in the leptomeninges (2–5%) and dura-mater(28).
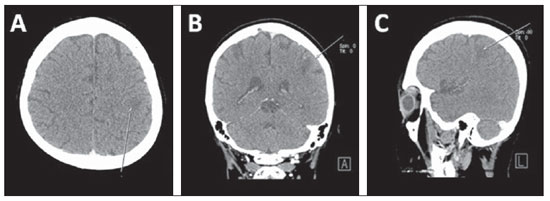
Figure 12. Brain metastasis. Axial (
A), coronal (
B) and sagittal (
C) MSCT images demonstrating brain metastasis in a breast cancer patient.
In most cases, brain metastasis manifests as isoattenuating or subtly hyperattenuating lesions, or even as hypoattenuating lesions with perilesional edema and intravenous contrast enhancement. Hemorrhages, cystic changes and necrosis are commonly seen in all types of metastatic tumors. However, there is no pathognomonic finding differentiating brain metastasis from primary malignant tumors from those originating from non-neoplastic conditions(29).
CONCLUSION
Despite the fact that MSCT is not the imaging method of choice to evaluate the breast parenchyma, its utilization allows the identification of relevant data in symptomatic patients, in patients with radiographic alterations and in patients who had lesions detected at abdominal ultrasonography, and also in the follow-up of response to chemotherapy treatment. The early diagnosis still remains as the best method to enhance the chances of cure for breast cancer.
REFERENCES
1. Brasil. Ministério da Saúde. Instituto Nacional de Câncer. Estimativa 2010: incidência de câncer no Brasil. Rio de Janeiro, RJ: INCA; 2009.
2. Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18–27.
3. Chala LF, Barros N. Avaliação das mamas com métodos de imagem. Radiol Bras. 2007;40(1):iv–vi.
4. Brasil. Instituto Nacional de Câncer. Controle do câncer de mama. Documento de consenso. Rio de Janeiro, RJ: INCA; 2004.
5. Soares Junior J, Fonseca RP, Cerci JJ, et al. Lista de Recomendações do Exame PET/CT com
18FFDG em Oncologia. Consenso entre a Sociedade Brasileira de Cancerologia e a Sociedade Brasileira de Biologia, Medicina Nuclear e Imagem Molecular. Radiol Bras. 2010;43:255–9.
6. Jung JI, Kim HH, Park SH, et al. Thoracic manifestations of breast cancer and its therapy. Radiographics. 2004;24:1269–85.
7. Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res. 2008;10:215.
8. Krengli M, Sacco M, Loi G, et al. Pulmonary changes after radiotherapy for conservative treatment of breast cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2008;70:1460–7.
9. Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24.
10. Christensen S, Pedersen L, Grijota M, et al. Incidence of interstitial pneumonitis among breast cancer patients: a 10-year Danish populationbased cohort study. Br J Cancer. 2008;98:1870–5.
11. Chen L, Gu Y, Leaw S, et al. Internal mammary lymph node recurrence: rare but characteristic metastasis site in breast cancer. BMC Cancer. 2010;10:479.
12. Bénard F, Turcotte E. Imaging in breast cancer: single-photon computed tomography and positron- emission tomography. Breast Cancer Res. 2005;7:153–62.
13. Yang SK, Cho N, Moon WK. The role of PET/CT for evaluating breast cancer. Korean J Radiol. 2007;8:429–37.
14. Cody HS 3rd, Urban JA. Internal mammary node status: a major prognosticator in axillary nodenegative breast cancer. Ann Surg Oncol. 1995;2:32–7.
15. Jager JJ, Keymeulen K, Beets-Tan RG, et al. FDGPET- CT for staging of high-risk breast cancer patients reduces the number of further examinations: a pilot study. Acta Oncol. 2010;49:185–91.
16. Chae BJ, Bae JS, Kang BJ, et al. Positron emission tomography-computed tomography in the detection of axillary lymph node metastasis in patients with early stage breast cancer. Jpn J Clin Oncol. 2009;39:284–9.
17. Takahashi M, Sasa M, Hirose C, et al. Clinical efficacy and problems with CT lymphography in identifying the sentinel node in breast cancer. World J Surg Oncol. 2008;6:57.
18. Yamashita K, Shimizu K. Evaluation of sentinel lymph node metastasis alone guided by three-dimensional computed tomographic lymphography in video-assisted breast surgery. Surg Endosc. 2009;23:633–40.
19. Bowen SL, Wu Y, Chaudhari AJ, et al. Initial characterization of a dedicated breast PET/CT scanner during human imaging. J Nucl Med. 2009;50:1401–8.
20. Avdalovic M, Chan A. Thoracic manifestations of common nonpulmonary malignancies of women. Clin Chest Med. 2004;25:379–90.
21. Seo JB, Im J, Goo JM, et al. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics. 2001;21:403–17.
22. Costelloe CM, Rohren EM, Madewell JE, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–14.
23. Koizumi M, Yoshimoto M, Kasumi F, et al. Comparison between solitary and multiple skeletal metastatic lesions of breast cancer patients. Ann Oncol. 2003;14:1234–40.
24. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93.
25. Diamond JR, Finlayson CA, Borges VF. Hepatic complications of breast cancer. Lancet Oncol. 2009;10:615–21.
26. Roach H, Whipp E, Virjee J, et al. A pictorial review of the varied appearance of atypical liver metastasis from carcinoma of the breast. Br J Radiol. 2005;78:1098–103.
27. Blake MA, McDermott S, Rosen MP, et al. Expert panel on gastrointestinal imaging. ACR Appropriateness Criteria
® suspected liver metastases. [online publication]. Reston, VA: American College of Radiology; 2011.
28. Wadasadawala T, Gupta S, Bagul V, et al. Brain metastases from breast cancer: management approach. J Cancer Res Ther. 2007;3:157–65.
29. Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–69.
1. PhD, Coordinator, Unit of Computed Tomography, Clínica de Medicina Nuclear e Radiologia de Maceió (MedRadiUS), Professor of Radiology and Imaging Diagnosis at Universidade Federal de Alagoas (UFAL), Maceió, AL, Brazil.
2. Titular Members of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), MDs, Clínica de Medicina Nuclear e Radiologia de Maceió (MedRadiUS), Maceió, AL, Brazil.
3. Graduate Students of Medicine, Monitors at Division of Radiology and Imaging Diagnosis, Faculdade de Medicina da Universidade Federal de Alagoas (UFAL), Maceió, AL, Brazil.
4. Graduate Students, Faculdade de Medicina da Universidade Federal de Alagoas, Maceió, AL, Brazil.
Mailing Address:
Dra. Christiana Maia Nobre Rocha de Miranda
Rua Hugo Corrêa Paes, 104, Farol
Maceió, AL, Brazil, 57050-730
E-mail: maiachristiana@globo.com
Received October 23, 2011.
Accepted after revision December 13, 2011.
Study developed at Clínica de Medicina Nuclear e Radiologia de Maceió (MedRadiUS), Maceió, AL, Brazil.
 Vol. 45 nº 2 - Mar. / Apr. of 2012
Vol. 45 nº 2 - Mar. / Apr. of 2012











