Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 45 nº 1 - Jan. /Feb. of 2012
Vol. 45 nº 1 - Jan. /Feb. of 2012
|
ORIGINAL ARTICLE
|
|
Endovascular repair of abdominal aortic aneurysm: a single-center results analysis |
|
|
Autho(rs): Eduardo Rafael Novero1; Patrick Bastos Metzger2; Fernanda Maria Resegue Angelieri2; Marcelo Bueno de Oliveira Colli2; Samuel Martins Moreira3; Nilo Mitsuru Izukawa4; Fabio Henrique Rossi5; Antonio Massamitsu Kambara6 |
|
|
Keywords: Aneurysm; Vascular graft; Atherosclerosis. |
|
|
Abstract: INTRODUCTION
Infrarenal abdominal aortic aneurysms (AAA) occur in 1% of the women and in 6% of men aged above 64. Aneurysm rupture is fatal in 80% to 90% of the cases, as pre-hospital deaths are taken into consideration(1). Considerable advances have been observed in the treatment of such a disorder. Undoubtedly, the endovascular technique, initially presented by Parodi and later developed by many researchers, has allowed the delivery of treatment for patients who would otherwise be at high risk if they were submitted to conventional open surgical treatment. Nowadays, with the accumulated experience and the development of safer and more flexible endografts, the endovascular treatment can be considered both for patients presenting surgical risk and those with anatomical characteristics favorable for conventional open surgical treatment(2). Endovascular repair is precisely indicated for patients presenting high surgical risk, the elderly, and for those cases where there are anatomical difficulties for the open surgery approach (hostile abdomen). The open surgical repair is considered effective and definitive, with graft failure rate of only 0.3% per year. However, such a technique presents non-negligible morbimortality rates, many times requiring prolonged recovery periods, sometimes reaching several months in the event of complications. On the other hand, the rate of reintervention in the endovascular treatment is not negligible, and is actually higher as compared with the open surgery approach(3—6). The present study is aimed at evaluating immediate and medium-term clinical outcomes in a series of patients submitted to endovascular repair of AAA in a reference center for cardiovascular medicine. MATERIALS AND METHODS Type of study The present retrospective, longitudinal and observational study was developed at Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil, in the period from January 2009 to July 2010, evaluating the outcomes of 102 patients submitted to endovascular repair of AAA. Inclusion and exclusion criteria The present study included both symptomatic and asymptomatic women and men with indication for endovascular repair of abdominal aortic aneurysms with > 55 mm in diameter for the male or 50 mm for the female patients, as well as for type I or type III endoleak repair in previously treated patients. Patients with proximal aneurysmal neck < 15 mm in length, presence of thrombus or calcification > 50% of the neck diameter, aneurysmal neck angulation > 65º, aortoiliac bifurcation > 90º, external iliac arteries diameter < 7 mm, serum creatinine levels > 2.0 mg/dl or creatinine clearance < 30 ml/min were excluded from the study (Figure 1). 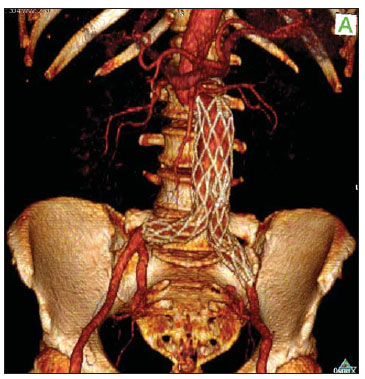 Figure 1. One-month tomographic follow-up of AAA repair. Aneurysmal neck with angulation of 60° and aortoiliac bifurcation with angulation of 65º. The patients who met the inclusion criteria were considered as having favorable anatomy and were consecutively referred for endovascular treatment. Anesthesia and/or cardiovascular risks were not taken into consideration for inclusion/exclusion of patients. In all of the cases, the diagnosis and therapeutic planning were based on computed tomography angiography, with preoperative arteriography being the optional diagnostic method. Surgical technique All the procedures were performed in the Hemodynamics Laboratory of Instituto Dante Pazzanese de Cardiologia, in São Paulo, SP, Brazil. The patients were treated under general inhalation anesthesia. Antimicrobial prophylaxis was performed with 1.5 g of cefuroxime, at the moment of the anesthetic induction. The preferred approach was through the common femoral artery, either unilateral or bilaterally according to the type of intervention or endograft to be deployed. In the cases where such an approach was not feasible the external iliac artery was approached by means of retroperitoneal access. The radiographic control was performed by an Artis flat panel apparatus (Siemens; Munich, Germany). The following devices were utilized: AnacondaTM (Vascutek, Terumo; Inchinnan, Scotland), AorfixTM (Lombard Medical Technologies PLC; Oxfordshire, UK), Apolo® (Nano; Florianópolis, SC, Brazil), EndurantTM (Medtronic; Minneapolis, MN, USA), Excluder® (Gore Medical; Flagstaff, AZ, USA), PowerLink System® (Endologix; Irvine, CA, USA), Talent® (Medtronic; Minneapolis, MN, USA), and Zenith Flex® (Cook Medical; Bloomington, IN, USA). All the patients underwent intraoperative arteriography. The immediate postoperative management of all the patients was carried out in the intensive care unit. Postoperative follow-up The patients were followed-up on an outpatient basis 15, 30, 180 and 360 days after the repair. After the first year, the follow-up was performed once a year. Computed tomography angiography was performed on the 1st and on the 12th month of the outpatient follow-up. Color Doppler ultrasonography was utilized in cases where CT angiography was contraindicated. Outcomes and definitions Primary and secondary outcomes were defined as follows: 1 — Technical success: when the objective of deploying the endograft in the affected area was achieved, with or without endoleaks or other events that could influence the favorable evolution of the aneurysm. 2 — Therapeutic success: when the endograft deployment occurred without endoleaks or other events that could affect the favorable evolution of the aneurysm. 3 — Perioperative mortality: number of deaths recorded within the first 30 days after the procedure. 4 — Annual mortality: number of deaths occurred within the 12-month follow-up. 5 — Periprocedural complications: a) intraoperative complications — those occurring in the hemodynamics room during the intervention; b) in-hospital complications — those complications occurring during hospital stay, out of the hemodynamics room and within 30 days after the intervention. 6 — Reintervention rate: interventions performed for the maintenance of satisfactory endograft function and exclusion of the aneurysmal sac, or for the resolution of complications associated with the intervention. Secondary outcomes 1 — Length of hospital stay: time elapsed between hospital admission and patient's discharge. 2 — Procedural time: time elapsed between anesthesia induction and surgical site closure. Endoleaks 1 — Initial or primary endoleak: those endoleaks originated during the initial procedure or diagnosed within the first 30 days. 2 — Secondary endoleaks: those diagnosed after 30 days from the initial procedure. RESULTS The follow-up comprised 102 patients submitted to infrarenal AAA repair. The mean age of the patients was 72 ± 9 years, and 79% of them were men. The predominant risk factor was hypertension in 100 patients (99%), 23 (22%) were diabetic patients, 23 (22%) were smokers, 47 (46%) were former smokers, and 51 (50%) patients presented dyslipidemia. Obesity with body mass index > 40 was present in 5 patients of the studied population. Amongst the comorbidities, a high incidence of ischemic cardiomyopathy (57.8%) was found, and in a lesser degree, chronic renal failure (14.7%). A significant percentage of patients with previous endovascular repair of aortic aneurysm were also found (12.7%) (Table 1). 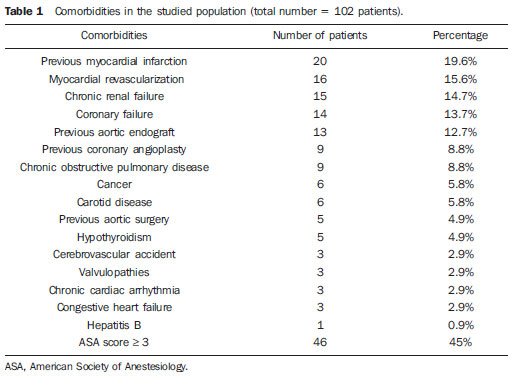 Most patients (76%) underwent elective treatment (asymptomatic abdominal aneurysms) while 24% were emergencially treated because of alarm symptoms of alarm symptoms (abdominal pain or tomographic signs of imminent aneurysm rupture). Presence of aneurysm was the indication for treatment in 88.2% of the cases, while management of endoleak after endovascular repair of AAA was the indication in 11.8% of the cases. The aneurysms morphology was predominantly fusiform, being found in 98 of the patients (96.1%). The remaining four patients had saccular aneurysms (3.9%). The types of treated aneurysms were classified according to the classification utilized in the EUROSTAR study, as follows: type A in 28 patients (27.4%); type B in 48 patients (47.0%); type C in 10 patients (9.8%); type D in 13 patients (12.7%); type E in 1 patient (1%). In 2 cases (2%), the indication was repair of abdominal aortic pseudoaneurysm (Figure 2). The mean diameter of the aneurysms was 61 ± 1.0 mm. 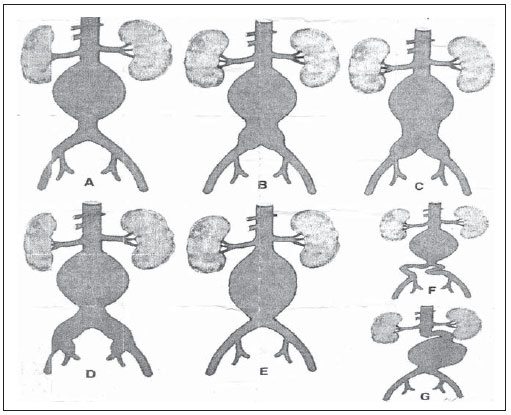 Figure 2. AAA classification –EUROSTAR study. The bifurcated endograft was the most utilized model, in 98% of the cases, and the mono-iliac endograft was utilized in only 2 patients (2%). The most utilized devices were the following: 23 (22.5%) Talent, 19 (18.6%) PowerLink System, 19 (18.6%) Zenith Flex, 17 (16.7%) Excluder, 9 (8.8%) Endurant, 8 (7.8%) Anaconda, 5 (4.9%) Aorfix, 2 (2%) Apolo. General inhalation anesthesia was utilized in all cases. Technical success was achieved in 97.1% of the cases. In 99 patients, the endograft could be deployed in the desired site. In three cases the endograft could not be advanced to the desired location because of technical or anatomic difficulties (presence of calcifications or tortuosity). Therapeutic success was achieved in 81% of the cases, that is to say, in 83 patients the endograft was deployed without endoleaks or other events that could affect a favorable outcome of the intervention. In 16 cases (15.7%) hypogastric artery embolization was required. Neither pelvic nor intestinal were observed in any case and erectile dysfunction was not reported during follow-up. The rate of complications was 19%, and the most frequent intraoperative complications were the following: active bleeding in the surgical site in 6 cases (5.8%), femoral lesion in 3 cases (2.9%) peripheral embolism in 3 cases (2.9%) and renal artery occlusion in 2 cases (1.9%), without progression to renal failure in these patients. In-hospital postoperative complications were the following: infections in 4r cases (3.9%) [2 cases of surgical wound infection (50%) and 2 cases of respiratory tract infection (50%)], and acute renal failure in 2 patients (1.9%). The perioperative mortality rate was 0.9%. One patient submitted to emergency aortic repair died because of aneurysm rupture. The total rate of endoleaks was 19.6%, with type I and III endoleaks being present in 16.6% of cases. No case of endograft migration was observed during follow-up. The rate of reintervention within one year was 18.8% resulting from management of types I and III endoleaks (Tables 2 and 3). The annual mortality rate observed during follow-up was 7.8%. One death occurred because of vascular causes (Figure 3). 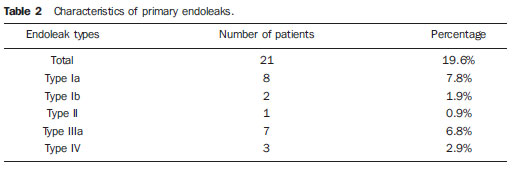 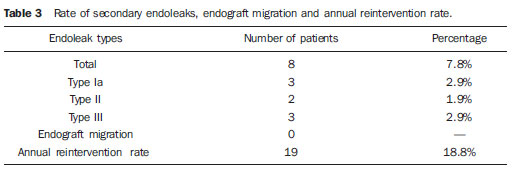 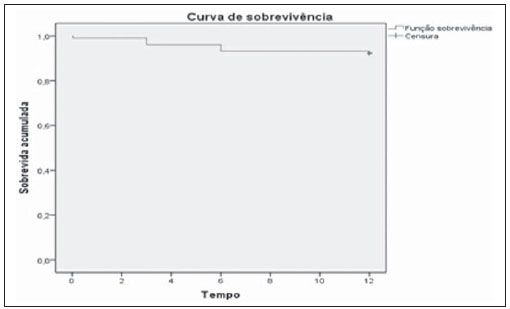 Figure 3. Mid-term survival. Probability of survival estimate (Kaplan-Meier method). Time expressed in months. The mean procedural time was 93 minutes (ranging from 65 to 130 minutes) and the mean hospital stay time was 5.2 days, with a variation of 9 days. DISCUSSION Over the past decade, the technique of endovascular repair of AAA has been developed as an alternative to open surgery. The impact of new devices and the two-decade experience from learning have led to the decrease in the operative mortality rate, as reported by several publications(7,8). The EVAR-I study(9) has demonstrated a decrease in perioperative mortality from 4.7% with open repair to 1.7% with endovascular repair, corresponding to a relative risk decrease of 65%. The EUROSTAR(10) study presented a 30-day mortality rate of 1.7% for minimally invasive treatment, while the DREAM(11) study presented a perioperative mortality rate of 4.6% for open surgery versus 1.2% for endovascular repair. Brazilian authors have reported a less significant decrease (6.5% versus 5.5% with conventional and endovascular repair, respectively)(12,13). In the authors' experience, the 30-day mortality rate was 0.9%. One patient died during intervention on a ruptured AAA. The operative mortality for ruptured AAA is above 40% for open surgery(14). Large randomized studies comparing open surgery versus endovascular repair in this type of medical emergency are yet to be completed, such as the case of the IMPROVE study(15). The annual mortality rate in the present study was 7.8% (Figure 3). There was one death due to vascular causes related to aneurysm rupture. Ischemic cardiomyopathy was most frequent comorbidity in the present study population, with a high number of patients presenting previous myocardial infarction, myocardial revascularization and coronary angioplasty (Table 1). Cardiac deaths were predominant, with highest prevalence of cardiogenic shock and cardiac arrest. The success of the endovascular technique depends on the continued exclusion of the aneurysmal sac(7). The positioning and deployment of the endograft for such a purpose is not always achievable, hence the importance of complementary preoperative studies such as CT angiography and arteriography to evaluate the feasibility of the procedure. Such studies allow the analysis of the aneurysmal diameter, the anatomic evaluation of the proximal and distal necks and the definition of iliac and femoral accesses, which constitute indispensable practices in this type of intervention. The rate of technical success was 97.1%, as in three patients the endograft could not be positioned and deployed because of underestimation of the degree of calcification and tortuosity of the access. In the EUROSTAR study(10) the endograft delivery system could not be appropriately advanced in 0.8% of the cases. The primary objective of every endovascular repair of aortic aneurysm is to prevent the aneurysm rupture and the potentially associated death. Other objectives include the reduction of the aneurysm size and the absence of endoleaks as markers of continued rupture risk. The rate of initial therapeutic success was 81%, measured as a function of the incidence of endoleaks in the primary procedures. In the EUROSTAR study(10) the incidence of initial endoleaks was the following: type I endoleak, 4.4%; type II, 9%; type III, 2.4%. In the present study population, the rate of initial endoleaks was 19.6% corresponding to: type Ia, 7.8% (8 cases); type Ib, 1.9% (2 cases); type II, 0.9% (1 case); type III, 6.8% (7 cases); type IV, 2.9% (3 cases). Such high rate of endoleaks was analyzed in detail. The percentage of types I and III endoleaks was 16%. In the population with type I endoleaks, 6 patients presented a minimum degree of leakage, which had spontaneously resolved at the time of the 30-day follow-up. In 3 cases of type III endoleak, the same phenomenon was observed. This fact sensibly reduces the rate of reintervention due to endoleak to 11%, a rate that is similar to other reports. As the aneurismal necks were evaluated in patients with type I endoleak, 4 patients were found with neck length between 15 and 17 mm, 2 patients with cone-shaped neck, and in 4 patients, the angulation was close to 65º. In one of their most important studies, Stanley et al. have analyzed the morphology of aneurysms as a predictor of endoleaks and complications, demonstrating that the presence of morphologies different from those recommended resulted in a four-fold increase in the occurrence of type I endoleaks, and the noncompliance with more than one recommendation multiplied such risk(16). The authors of the present study have undoubtedly explored the limits of the therapeutic endovascular intervention. According to May et al., intraoperative complications may be observed in approximately 20% of the patients treated by endovascular approach. Some complications are inversely related to the length of the aneurismal neck, that is, the shorter the length of the neck, the more difficult it is to deploy the endovascular device(17). Also the greater the number of comorbidities, the higher the probability of intra- and postoperative adverse events. The DREAM study, which has compared the operative mortality and complications in surgical treatments with endovascular repair within 30 days, concluded that the endovascular repair is preferable because of the lower rates of mortality and complications, and also because of the significant reduction of the incidence of systemic complications(11). In the EVAR-I study, the rate of complications was 35%, while in the EVAR-II it was 33%(9,18). In the present casuistry, the total rate of complications was 19% within the 30-day periprocedural period. Intraoperative bleeding in the surgical site was present in 6 cases (in 5 cases, at the level of the inguinal region, and 1 case of retroperitoneal hematoma), and femoral injury in 3 cases. Such complications were the ones most frequently observed in the present study. Undoubtedly, the profile of the utilized materials, in association with unfavorable surgical practices, does influence such rate. Reitervention is performed in order to maintain the endografts function and, in some cases, for the resolution of remote complications. According to Sampram et al.(19), main indications for reintervention are the following: a) endoleaks; b) endograft migration; c) occlusion of iliac branches; d) postoperative hemorrhage; e) renal occlusion. Less frequently, distal embolization, infection and aneurysm rupture are observed. In the present study, most of the reinterventions occurred for endoleaks related to the initial procedure (11%) and, to a lesser degree, for secondary endoleaks (7.8%) observed after the 30-day periprocedural period. The annual rate of reintervention was 18.8%. The EVAR-I study presented a reintervention rate of 20% in a four-year follow-up; and in the EVAR-II study, such a rate was 26%. On the other hand, the DREAM and EUROSTAR studies presented rates of 14.3% and 13.5%, respectively, in a three-year follow-up(9—11,18). The DREAM study has shown that the rate of reintervention was threefold higher in the first nine months for the endovascular repair as compared with open surgery, with such rates becoming equal at the two-year follow-up(11). In such a study, the main reason for reintervention was the onset of types I and II endoleaks. Drury et al. have reported a reintervention rate for initial endoleak of 17.5%, with 3.5% being type I endoleaks and 14%, type II endoleaks. At 12 months, the endoleak rate was 21%, with increase in the number of type I endoleaks (6.8%) and type III endoleaks, and decrease in the number of type II endoleaks. The annual rate of secondary reintervention was 16.2%(20). In the present casuistry, the percentages of secondary endoleaks were the following: type I, 2.9%; type II, 1.9%; type III, 2.9%; maintaining the high indices of endoleak types I and III as causes for reintervention during one-year follow-up. In the EUROSTAR study, with a five-year follow-up, the main indication for reintervention was the type II endoleak, followed by the type III(10). One should highlight that, in the present study, the patient who presented type II endoleak did not undergo reintervention since no aneurysmal sac increase was detected at CT angiography, as well as in the cases of type IV endoleaks, where no further endoleak was observed during tomographic follow-up, thus being thus considered as spontaneous resolution. CONCLUSION The results obtained by means of endovascular repair of AAA are associated with low indices of complications in the short and medium-terms. Such rates of complication and reintervention are similar to those obtained in other Brazilian and international reference centers. Such results support the performance of such procedure in patients with suitable anatomy. REFERENCES 1. Mastracci TM, Cinà CS; Canadian Society for Vascular Surgery. Screening for abdominal aortic aneurysm in Canada: review and position statement of the Canadian Society for Vascular Surgery. J Vasc Surg. 2007;45:1268—76. 2. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491—9. 3. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437—44. 4. Hallett JW Jr, Marshall DM, Petterson TM, et al. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J Vasc Surg. 1997;25:277—84. 5. Johnston KW. Nonruptured abdominal aortic aneurysm: six-year follow-up results from the multicenter prospective Canadian aneurysm study. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg. 1994;20:163—70. 6. Silvestre JMS, Motta F, Sardinha WE, et al. Endovascular treatment of infrarenal abdominal aortic aneurysm in patients with favorable anatomy for the repair — initial experience in a university hospital. J Vasc Bras. 2011;10:31—9. 7. Matsumura JS, Brewster DC, Makaroun MS, et al. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 2003;37:262—71. 8. Blankensteijn JD, de Jong SE, Prinssen M, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398—405. 9. Greenhalgh RM, Brown LC, Kwong GP, et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364:843—8. 10. Harris PL, Vallabhaneni SR, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32:739—49. 11. Prinssen M, Verhoeven EL, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607—18. 12. Mendonça CT, Moreira RCR, Timi JRR, et al. Comparação entre os tratamentos aberto e endovascular dos aneurismas da aorta abdominal em pacientes de alto risco cirúrgico. J Vasc Bras. 2005;4:232—42. 13. Mendonça CT, Moreira RCR, Carvalho CA, et al. Tratamento endovascular de aneurismas da aorta abdominal em pacientes de alto risco cirúrgico. J Vasc Bras. 2009;8:56—64. 14. Pae SJ, Carr JA. Ruptured abdominal aortic aneurysm in community practice: age and operative variables predict survival. Am Surg. 2007;73:912—6. 15. IMPROVE Trial, Powell JT, Thompson SG, Thompson MM, et al. The immediate management of the patient with rupture: open versus endovascular repair (IMPROVE) aneurysm trial — ISRCTN 48334791 IMPROVE trialists. Acta Chir Belg. 2009;109:678—80. 16. Stanley BM, Semmens JB, Mai Q, et al. Evaluation of patient selection guidelines for endoluminal AAA repair with the Zenith Stent-Graft: the Australasian experience. J Endovasc Ther. 2001;8:457—64. 17. May J, White GH. Endovascular treatment of aortic aneurysm. In: Rutherford RB, editor. Vascular surgery. 5th ed. Philadelphia, PA: WB Saunders; 2000. p. 1281—95. 18. EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365:2187—92. 19. Sampram ES, Karafa MT, Mascha EJ, et al. Nature, frequency, and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2003;37:930—7. 20. Drury D, Michaels JA, Jones L, et al. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysm. Br J Surg. 2005;92:937—46. 1. MD, Interventional Cardiologist, Foreign Trainee at Centro de Intervenções Endovasculares (CIEV), Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil. 2. MDs, Vascular Surgeons, Trainees at Centro de Intervenções Endovasculares (CIEV), Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 3. MD, Vascular and Endovascular Surgeon, Assistant at Division of Medical Radiology, Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 4. PhD, Surgeon-in-Chief, Medical Division of Vascular Surgery, Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 5. PhD, MD, Physician Assistant, Medical Division of Vascular Surgery, Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. 6. PhD, Titular Member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), Head of Division of Medical Radiology, Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. Mailing Address: Dr. Patrick Bastos Metzger Centro de Intervenções Endovasculares (CIEV) Avenida Doutor Dante Pazzanese, 500, Vila Mariana São Paulo, SP, Brazil, 04012-909 E-mail: patrickvascular@gmail.com Received September 12, 2011. Accepted after revision November 8, 2011. Study developed at Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554