INTRODUCTION
Langerhans cell histiocytosis (LCH), previously known as histiocytosis X, is a rare disease with unknown causes, in spite of several evidences suggesting immunological origin(1).
The disease is characterized by the infiltration of Langerhans cells in several organs, although the simple finding of such cells is not pathognomonic of the disease. Langerhans cell has an accessory immune function and its most peculiar characteristics are the cytoplasmic Birbeck granules, and the glycoprotein CD1a, which is a surface marker identified by immunohistochemistry(2,3).
The disease may present in the systemic (or multifocal) form, most frequent in children, or localized (unifocal), common in adults. In LCH, lungs involvement occurs most frequently in young adults, with a slight male predominance, with most patients being smokers. Extrapulmonary lesions, particularly lytic bone lesions, may be observed in association with lung changes(4,5).
Pathologically, pulmonary LCH is characterized by the presence of destructive granulomatous lesions containing Langerhans cells, with nodular aspect and preferentially located next to the terminal bronchioles. Cysts appear as the disease progresses, but the exact mechanism of development of such lesions is still to be determined is still to be determined(6,7).
Nodules, and particularly cysts, are the most expressive radiological manifestations of pulmonary LCH. The initial investigation of such lesions is done by means of plain chest radiography, which in a high number of cases allows satisfactory evaluation. However, the lack of specificity of the method represents a limitation to its application in the diagnosis of such a disease(8).
The findings observed at high resolution computed tomography (HRCT) reflect the morphological changes caused by the disease with greater reliability than conventional radiology, being therefore the most effective method for identifying and characterizing pulmonary interstitial changes(9).
In most of cases, the definitive diagnosis of LCH has been made by means of open lung biopsy(10). However, in many cases, HRCT can be capable to determine the diagnosis of pulmonary LCH(11). The definition of tomographic patterns characteristic of the disease is of great clinical relevance as it allows the imaging diagnosis, avoiding open lung biopsy, which is an invasive and expensive procedure.
MATERIALS AND METHODS
In the present study, HRCT images of eight patients with diagnosis of pulmonary LCH were retrospectively analyzed.
Seven patients underwent follow-up at the Clinic of Diffuse Interstitial Pneumopathies of the Unit of Pneumology at Hospital Universitário Pedro Ernesto - Universidade do Estado do Rio de Janeiro, and one patient at the Unit of Pneumology of Hospital Universitário Clementino Fraga Filho - Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil.
At the time of diagnosis, the patients’ ages ranged between 18 and 61 years (mean = 35.4 and median = 39.5 years). The extreme aged (18 and 61 years) patients were women. Among the eight patients included in the study, four (50%) were men and four (50%) were women.
Seven (87.5%) of the patients reported a history of smoking. Two of such patients had quit smoking and one of the patients reported heavy smoking. Such a (female) patient presented high levels of CD1a-positive cells in bronchoalveolar lavage.
Four of the patients (50%) presented bone lesions associated with lung changes. One of such patients presented ulcerative skin lesions, besides bone lesions. Bone lesions were located at the ulna, mandible, skull and spine.
Cough and dyspnea were symptoms of the disease in all the patients. Three patients described their cough as dry, and one as being mucoid. In the other four cases no reference was made to expectoration. The third most frequent clinical manifestation was pneumothorax, observed in 37.5% of the patients.
Data regarding the disease progression were available in the seven of the eight studied patient records. Clinical stabilization was observed in four patients, while in three cases the disease progressed with worsening of respiratory symptoms as well as of radiological features.
The diagnosis was achieved by means of open lung biopsy in three patients, transbronchial biopsy in one patient, and immunohistochemistry (increased CD1a-positive cells levels) in two patients. In two patients the diagnoses were based on typical clinical and tomographic findings, associated with characteristic bone lesions. The patients’ identification and clinical data were obtained from a review of their medical records.
The following findings were analyzed in the evaluation of the chest radiographic images: presence of nodules, cysts, reticular infiltrate, emphysematous changes and lung volume changes, as well as distribution of lesions throughout the lung parenchyma and distribution symmetry.
In the evaluation of HRCT images, the lesions were defined according to the Glossary of Terms for CT of the Lungs(12):
Nodules
Nodules were considered as “rounded, at least partially well delimited opacities, with a maximum of 3 cm in diameter”(4).
Nodules size evaluation was done by means of a method modified by Lacronique et al.(13). The nodular lesions were characterized according to size, in nodules with up to 5 mm, nodules between 5 and 10 mm, and macronodules above 10 mm.
Excavated nodules corresponded to those with a central excavation, in which the solid component predominated over the cystic component(14).
Cysts
Cysts corresponded to “round parenchymal spaces, with well defined walls, usually containing air, when located in the lung, but without associated lung emphysema”(12).
Cysts were classified according to their size as follows: small (< 10 mm), medium (10-20 mm) and large (> 20 mm). Their walls were classified into thin (< 1 mm), and thick (> 1 mm). Cystic lesions were classified according to their shapes, into round, lobulated, confluent and with septa.
Lesions distribution
As regards distribution over the lung parenchyma, the lesions were classified according to their predominance, in the upper, middle, lower, and extreme lower regions of the lung. The upper region was considered as being the segment from the apex to the aortic arch; the middle region, from the aortic arch to the inferior pulmonary vein; the lower region, from the inferior pulmonary vein to the hemidiaphragm domes; and extreme lower region, the costophrenic recesses.
Evaluation was made on whether the lesions predominated in central or peripheral zones of the lungs, and on whether or not there was symmetry in the lesions distribution between the two pulmonary fields.
Associated changes
The following findings corresponding to changes associated with nodules and cysts were analyzed: linear opacities, ground-glass attenuation, interlobular septal thickening, pleural effusion, lung parenchyma distortion, pneumothorax and emphysematous changes.
RESULTS
High-resolution computed tomography images of eight adult patients diagnosed with pulmonary LCH were retrospectively evaluated.
Radiological findings
Chest radiography
Chest radiographs of six patients were available for evaluation. One of the six radiographs was considered as being normal, but HRCT of this same female patient revealed the presence of sparse thin walled cysts. In three cases, cysts could be identified at radiography, and HRCT of these patients demonstrated thick-walled cysts. In the other two cases, where radiography failed to identify cystic lesions, HRCT demonstrated only thin-walled cysts. Reticular infiltrate was observed in four cases, and reticulonodular infiltrate in one case.
Small nodules were clearly individualized at chest radiography of only two patients, although HRCT has demonstrated nodular lesions in five patients. In one case where nodules were identified at radiography, HRCT demonstrated lesions larger than 5 mm. Among the five radiographs demonstrating changes, three had a good correlation with HRCT as lesions distribution are considered.
High-resolution computed tomography
In the evaluation of the HRCT images, nodules were observed in six (75%) of the eight cases. In four cases, the quantity of nodular lesions was small and in two cases, moderate. The nodules presented predominantly peripheral distribution in six patients (Figure 1). In one case, the nodules were randomly distributed throughout the pulmonary parenchyma.
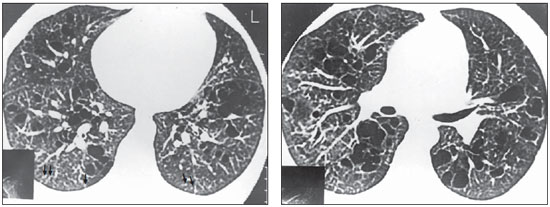
Figure 1. HRCT. Nodules < 5 mm, peripherally distributed in the lungs, particularly in the posterior regions (arrows). Thin-walled cysts, some of them confluent, with septa inside, predominating in the central zones of the pulmonary parenchyma (case 4).
The six patients presented nodules smaller than 5 mm in diameter, but in one case nodules between 5 mm and 10 mm were also observed in association with smaller nodules. No patient presented nodules > 10 mm. Scarce excavated nodules were identified in case 1 (Figure 2). The typical star-shaped nodule was observed in only one patient (Figure 2). In all of the cases, nodular lesions were observed in association with cysts.
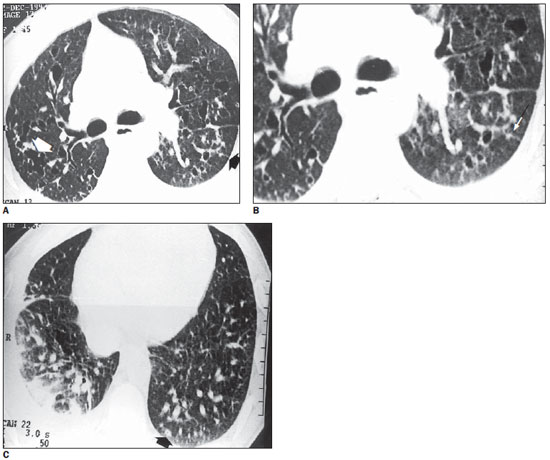
Figure 2. HRCT. On A, at left, multiple thin-walled cysts, some of them confluent, determining bizarre shapes (white arrow) associated with nodules with irregular contours (black arrow). On B, magnification of A with greater detail of the star-shaped nodule. On C, presence of sparse cysts and nodules in the lower lobes. Note the presence of nodule with small central excavation in the posterior left basal region (arrow). Pleural effusion at left. Vessels with increased diameter (case 1).
At HRCT, all the patients presented small cysts (< 10 mm) in variable quantities (Figures 1, 2, 3, 4 and 5). Cysts with diameters between 10 and 20 mm were identified in six patients (75%), with small quantity in three cases, moderate quantity in two cases and large quantity in one case. In three patients (37.5%), few cysts > 20 mm were observed. In 75% of the cases, cysts of different sizes were observed in a single patient (Figure 1).
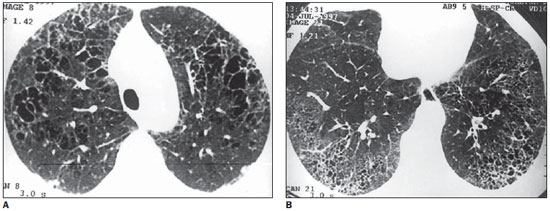
Figure 3. HRCT. On A, thin-walled round cysts of several sizes with fine or confluent septa. In the peripheral zone, nodules and interlobular septal thickening (anterior, at right) are observed. On B, presence of small cysts with similar sizes and peripheral distribution suggesting honeycombing, involving the posterior regions of the lower lobes. Note the presence of a peripheral nodule at right (case 5).
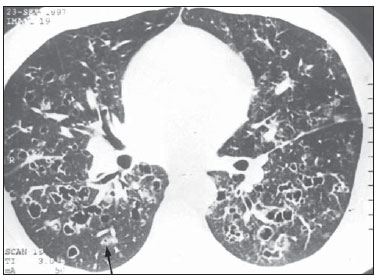
Figure 4. HRCT. Round, lobulated, confluent and bizarre cysts, with varied wall thicknesses. Sometimes, it becomes difficult to differentiate small thick-walled cysts from excavated nodules (arrow) (case 1).
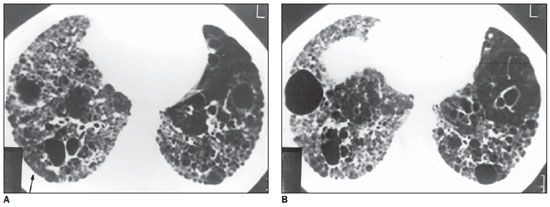
Figure 5. HRCT. Cysts with varied diameters, some of them with thick walls. Also, thick-walled dilated bronchi, and areas of architectural distortion are observed. On A, note the presence of small nodules (arrow) in the right, posterior, basal region. On B, an isolated nodule is observed, anteriorly located at left, with involvement of the costophrenic recesses (case 3).
Thin-walled cysts (< 1 mm) were observed on all the HRCTs. In four cases, however, a small number of thick-walled cysts (> 1 mm) were observed in association with thin-walled cysts (Figure 4).
Round cysts were found in all the patients. In 62.5% of the cases different shapes of cysts were observed in a single patient. Associations of round and confluent cysts (50%) and round and lobulated cysts (50%) were the most frequently observed. Association of round, confluent cysts, and cysts with thin septa inside was found in 37.5% of the cases (Figure 3). Cysts with a bizarre appearance were observed in two patients, being apparently secondary to the confluence of several cysts (Figure 2).
The combination of cysts of several sizes and nodular lesions smaller than 5 mm was the most frequent finding in the present study, being observed in 6 (75%) of the eight patients (Figures 1, 2, 3 and 4).
Other changes associated with cysts and nodules were observed at HRCT in six patients. The most frequent ones were interlobular septal thickening, linear opacities, ground-glass attenuation, pulmonary parenchyma distortion and pleural reaction, each occurring in three patients (Figure 5).
Two patients with advanced disease presented honeycombing and bronchiectasis. Small peripheral cystic lesions, suggesting honeycombing, were also observed in one case (Figure 3).
Pleural effusion was observed at HRCT only in case 1, and pneumothorax only in case 8, although both findings have been diagnosed by clinical investigation and by means of plain radiography in the highest number of cases. Unusual emphysematous changes and lymph node enlargement were observed in two patients.
In two patients, the pulmonary parenchyma adjacent to the lesions was preserved. In one of such cases, only thin-walled cysts were observed, with a tomographic appearance indistinguishable from pulmonary lymphangioleiomyomatosis (Figure 6).
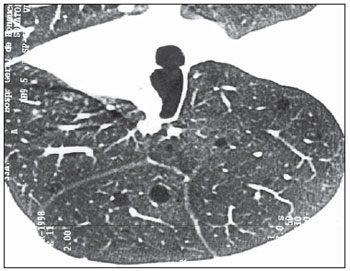
Figure 6. HRCT. Rare and sparse thin-walled cysts throughout the lungs, with preserved pulmonary parenchyma intermingled with lesions. No evidence of nodules is observed (case 6).
In the eight studied cases, the lesions affected the upper and middle regions of the lungs. In seven patients (87.5%), the lesions also involved the lower region of the lungs, although in six (75%) cases the lesions were observed predominantly in the upper and middle regions. On the HRCT of one patient, the lesions presented a homogeneous distribution in the upper, middle and lower lung regions. In 25% of the patients, involvement of the most inferior regions of the lung, including the costophrenic recesses, was observed (Figure 5).
Homogeneous lesions distribution in the central and peripheral regions of the lung was observed in five (62.5%) of the eight studied patients. In the remaining cases (37.5%), the lesions predominated in the central portions of the pulmonary parenchyma (Figure 7). Cystic lesions were most frequently observed in the central portions, and nodules predominated in the peripheral regions.
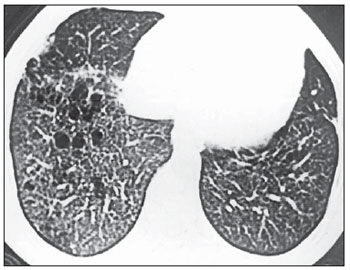
Figure 7. HRCT. Confluent cysts predominating in the central zones of the lung and small nodules in the peripheral regions. Note the asymmetry between the lungs, with predominance of lesions at right (case 2).
Pleuropulmonary fields were symmetrically affected by lesions in seven (87.5%) of the eight patients. Only one of the cases presented asymmetry in the distribution of lesions (Figure 7).
DISCUSSION
In the present study, two major limitations were observed. The first one was related to the low incidence of LCH and the low frequency of pulmonary involvement by such a disease. The reduced number of cases allows only studies limited to small groups, making the development of prospective studies unfeasible.
The second limitation is related to the confirmation of pulmonary LCH diagnosis. The definitive diagnosis is achieved by means of open lung biopsy(10). Results of immunohistochemistry testing of bronchoalveolar lavage, particularly the presence of increased CD1a-positive cells levels, are highly suggestive of the disease, but must be associated with other clinical and radiological findings for diagnosis(15-17). The association of clinical history and typical radiological findings has been accepted by pneumologists for the diagnosis in cases where the patient refuses the lung biopsy approach and where BAL has not demonstrated significant changes. Thus, for many patients, the diagnosis is actually a supposed diagnosis, a fact that makes the utilization of such cases in scientific studies quite troublesome.
In the present study, four (50%) of the patients were men and four (50%) were women. Pulmonary LCH was historically considered as a disease typically found in men. However, in 1983, Colby & Lombard(6) reviewed 13 studies approaching the disease distribution by gender and found male predominance in ten studies, female predominance in two studies and equal incidences in one study, with a total of 320 patients -185 men (57.8%) and 135 women (42.2%). Notwithstanding the characterization of this male predominance, the current trend in the literature leans towards not considering pulmonary LCH a man’s disease. Thus, in spite of the small sample, the present study data corroborate those reported by other studies(18.19).
Similarly to data reported in the literature(6), the ages of the patients at the time of diagnoses ranged between 18 and 61 years (mean 35.4 years).
Extrapulmonary involvement was observed in four patients (50%), three of them with bone lesions and one patient with associated bone and skin lesions. The involvement of other organs besides the lung was greater than that reported in the literature. Colby(4) reports that less than 15% of adult patients with lung pulmonary LCH will present extrapulmonary lesions or disseminated disease. Such a fact is possibly a consequence of the difficulty in diagnosing interstitial diseases of the pulmonary parenchyma. In this context, the presence of extrapulmonary disease, particularly the finding of bone lesions, certainly facilitates the diagnosis of LCH. In the present study, two of the patients were diagnosed by means of the association of lung changes and bone lesions.
Information regarding the disease progression was obtained from seven patients. Stabilization of clinical conditions was observed in 57% of the patients, and progression with worsening of respiratory symptoms, in 43%. Such data contrast with those observed in several other studies, which report a more favorable progression for the pulmonary presentation of LCH(20). Of the 60 patients from whom Friedman et al.(19) obtained data information the disease progression, 16 (26.7%) were asymptomatic and remained so, 39 (65%) presented stabilization or symptoms remission, and only 5 (8.3%) experienced progression of the disease.
Chest radiographs of six patients in the present study were available and the most frequent findings were reticulation, observed in four of the six cases (66.7%), and cysts in three of the six cases (50%). In three cases, HRCT was capable of demonstrating that the reticulation observed at plain radiography corresponded in part to thin-walled cysts. Well defined cysts observed at radiography corresponded to thick-walled cysts at tomography. Radiography, contrary to HRCT, was not capable of demonstrating characteristics on the cysts shape, which are of great value in the imaging diagnosis of pulmonary LCH.
In the present study, a good correlation was not observed between changes observed at chest radiography and those observed at HRCT. In only two out of the six cases (33.3%), the association of nodules and cysts, that is the most characteristic change in pulmonary LCH, could be observed at plain radiography. Grenier et al.(11) have reported similar findings in their study of 17 cases of pulmonary LCH, diagnosing high probability of the disease in 40% of the chest radiographs.
In the present study, nodules were observed at HRCT of six (75%) of the eight patients, a frequency similar to that reported in the literature(21). However, the presence and quantity of nodules varied with the stage of the disease. In one of the cases where nodules were not observed, the patient presented the disease in advanced stage and the main tomographic findings corresponded to end-stage lung disease. The typical star-shaped nodules are observed with higher frequency at the earliest phases of the disease, and in the present study they were not common (12.5%).
Cysts were the most frequent HRCT finding in patients with pulmonary LCH, and could be observed in all the patients. As regards cyst sizes, there was a predominance of small cysts (< 10 mm) that were present in 100% of the HRCTs. In 75% of the cases, cysts with diameters between 10 and 20 mm were observed, and in 37.5% lesions > 20 mm were identified. In 75% of the cases cysts of different sizes were identified in a single patient. Such data are in agreement with those commonly reported in the literature(14).
Thick-walled cysts were observed in 50% of the cases in the present study. In spite of the presence of thick-walled cysts observed only in small quantities in each of the cases, the frequency of their occurrence was higher than that reported in the literature. Bonelli et al.(22) have not found thick-walled cysts in ten studied cases. It should be highlighted, however, that even at HRCT it can be difficult to characterize cystic lesions, and sometimes the differentiation between excavated nodules and ectatic bronchi with small thick-walled cysts may be not obvious.
In the present study, the most frequent cyst shape was the round one, observed in all the patients. In 62.5% of the cases, lobulated, confluent or cysts with septa were observed, several of them determining bizarre shapes. Such atypical appearances of cystic lesions are very particular of the disease(23).
In the present study, the association of cysts of different sizes and shapes with nodular lesions smaller than 5 mm was observed in 75% of the patients. Such an association is highly suggestive of pulmonary LCH(11).
Although nodules have been observed in six cases (75%), a significant predominance of cystic lesions was observed in all the patients. In two cases (25%) nodules were not observed. However, in one case the tomographic findings were uncommon for LCH, with only small thin-walled round cysts being observed, without any change in the adjacent pulmonary parenchyma; and in the other case the disease was at a late stage, where the presence of nodules is not usual.
High-resolution computed tomography of six patients demonstrated other findings in association with cysts and nodules. The most frequent ones were interlobular septal thickening, linear opacity, ground-glass attenuation, pulmonary parenchyma distortion and pleural reaction, each one of them occurring in three patients. Pleural reaction is not a frequent CT finding in pulmonary LCH patients and, at microscopy, it may be the expression of reactive eosinophilic pleuritis(4). In the present study, one of the patients had a history of pleural effusion. In the other two cases, no history of pleural diseases was found. However, in the Brazilian population, the finding of pleural reaction must be carefully considered, because of the high prevalence of such a change as a consequence of tuberculosis. In two patients, honeycombing was observed at HRCT, and in both cases the disease was at a late stage of progression.
In pulmonary LCH, the involvement of costophrenic recesses is not common. Bonelli et al.(22) have found lesions in such a site in one (10%) of their ten cases. Several studies have highlighted such characteristic as being relevant in the differential diagnosis of LCH with other interstitial diseases(23,24). In the present study, a higher percentage of costophrenic recesses involvement was observed as compared with that reported in the literature (25%). However, it should be highlighted that, in case 5, the characteristic lesions of pulmonary LCH were observed only in the two upper regions of the lungs, with the changes in the lower lobes being similar to those found in pulmonary fibrosis, with lesions (honeycombing) located at the peripheral regions of the lungs, particularly in the posterior areas. The lesions were homogeneously distributed throughout the central and peripheral zones of the pulmonary parenchyma in five (62.5%) of the eight cases. In the remaining cases (37.5%), the lesions were predominantly located in the central zones of the lungs. However, such finding has not been usually reported. Moore et al.(21) have not observed differences in the horizontal distribution of the lesions, and Brauner et al.(14) and Lieberman et al.(24) have described predominance in the peripheral regions.
In one patient, it was possible to correlate HRCT findings with histopathological findings. Several stages of the disease progression could be observed. Early changes such as areas of cellular infiltrate, characterized particularly by the presence of lymphocytes and Langerhans cells, were identified in multiple sites.
As the disease progresses, the infiltrate organizes into granuloma, in typically peribronchiolar location. The infiltrate extension towards the alveolar septa determines the irregular appearance of the granuloma, which is visualized at CT as a star-shaped nodule.
Destruction of the pulmonary parenchyma with development of cysts occurs as the disease progresses. The characterization of cysts is difficult at microscopy, as they tend to collapse as the biopsy specimen is sectioned. However, areas of pulmonary parenchyma destruction are commonly identified.
The present study confirms that HRCT is the best imaging method for the evaluation of changes observed in the pulmonary presentation of LCH, for allowing better visualization and characterization of cystic and nodular lesions. The association of such lesions is not commonly observed in other lung diseases and their presence is strongly suggestive of pulmonary LCH.
REFERENCES
1. Osband ME. Histiocytosis X. Langerhans’ cell histiocytosis. Hematol Oncol Clin North Am. 1987;1:737-51.
2. Komp DM. Langerhans cell (eosinophilic) granulomatosis. In: Cecil RL, Plum F, Bennett JC, editors. Cecil textbook of medicine. 20th ed. Philadelphia, PA: Saunders; 1996. p. 955-6.
3. Reynolds HY. Langerhans cell granulomatosis. In: Fauci AS, Braunwald E, Isselbacher KJ, et al., editors. Harrison’s principles of internal medicine. 14th ed. New York, NY: McGraw-Hill; 1998. p. 1465.
4. Colby TV. Pulmonary histiocytosis X. In: Hasleton PS, editor. Spencer’s pathology of the lung. 5th ed. New York, NY: McGraw-Hill; 1996. p. 767-801.
5. Valeyre D, Soler P, Hance A. Langerhans' cell granulomatosis. In: Bone RC, Dantzker DR, George RB, et al., editors. Pulmonary and critical care medicine on CD-ROM. St. Louis, MO: Mosby-Year Book; 1996.
6. Colby TV, Lombard C. Histiocytosis X in the lung. Hum Pathol. 1983;14:847-56.
7. Soler P, Kambouchner M, Valeyre D, et al. Pulmonary Langerhans’ cell granulomatosis (histiocytosis X). Annu Rev Med. 1992;43:105-15.
8. Naidich DP. High resolution computed tomography of cystic lung disease. Semin Roentgenol. 1991;26:151-74.
9. Nakata H, Kimoto T, Nakayama T, et al. Diffuse peripheral lung disease: evaluation by high-resolution computed tomography. Radiology. 1985;157:181-5.
10. Smith CM. Eosinophilic granuloma. In: Bone RC, Dantzker DR, George RB, et al., editors. Pulmonary and critical care medicine on CD-ROM. St. Louis, MO: Mosby-Year Book; 1996.
11. Grenier P, Valeyre D, Cluzel P, et al. Chronic diffuse interstitial lung disease: diagnostic value of chest radiography and high-resolution CT. Radiology. 1991;179:123-32.
12. Austin JHM, Müller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996;200:327-31.
13. Lacronique J, Roth C, Battesti J-P, et al. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax. 1982;37:104-9.
14. Brauner MW, Grenier P, Mouelhi MM, et al. Pulmonary histiocytosis X: evaluation with high-resolution CT. Radiology. 1989;172:255-8.
15. Arico M, Egeler RM. Clinical aspects of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12:247-58.
16. Brauner MW, Grenier P, Tijani K, et al. Pulmonary Langerhans cell histiocytosis: evolution of lesions on CT scans. Radiology. 1997;204:497-502.
17. Refabert L, Rambaud C, Mamou-Mani T, et al. Cd1a-positive cells in bronchoalveolar lavage samples from children with Langerhans cell histiocytosis. J Pediatr. 1996;129:913-5.
18. Weber WN, Margolin FR, Nielsen SL. Pulmonary histiocytosis X. A review of 18 patients with reports of 6 cases. Am J Roentgenol Radium Ther Nucl Med. 1969;107:280-9.
19. Friedman PJ, Liebow AA, Sokoloff J. Eosinophilic granuloma of the lung. Clinical aspects of primary pulmonary histiocytosis in the adult. Medicine (Baltimore). 1981;60:385-96.
20. Malpas JS. Langerhans cell histiocytosis in adults. Hematol Oncol Clin North Am. 1998;12:259-68.
21. Moore AD, Godwin JD, Müller NL, et al. Pulmonary histiocytosis X: comparison of radiographic and CT findings. Radiology. 1989;172:249-54.
22. Bonelli FS, Hartman TE, Swensen SJ, et al. Accuracy of high-resolution CT in diagnosing lung diseases. AJR Am J Roentgenol. 1998;170:1507-12.
23. Webb WR, Müller NL, Naidich DP. High-resolution CT of the lung. 2nd ed. Philadelphia: Lippincott-Raven; 1996.
24. Lieberman PH, Jones CR, Steinman RM, et al. Langerhans cell (eosinophilic) granulomatosis. A clinicopathologic study encompassing 50 years. Am J Surg Pathol. 1996;20:519-52.
1. MD, Unit of Radiology, Hospital Universitário Clementino Fraga Filho (HUCFF) da Universidade Federal do Rio de Janeiro (UFRJ), and Instituto D’Or para Pesquisa e Educação, Rio de Janeiro, RJ, Brazil.
2. Assistant Professor of Pneumology, Universidade do Estado do Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil.
3. Assistant Professor of Radiology, Universidade do Estado do Rio de Janeiro (UERJ), MD, Unit of Radiology, Hospital Universitário Clementino Fraga Filho (HUCFF) da Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
Mailing Address:
Dra. Rosana Souza Rodrigues
Rua Diniz Cordeiro, 30, Botafogo
Rio de Janeiro, RJ, Brazil, 22281-100
E-mail: rosana.souzarodrigues@gmail.com
Received March 30, 2011.
Accepted after revision May 25, 2011.
Study developed at Unit of Radiology, Hospital Universitário Clementino Fraga Filho (HUCFF) da Universidade Federal do Rio de Janeiro (UFRJ), and at Unit of Pneumology, Hospital Universitário Pedro Ernesto (HUPE) da Universidade do Estado do Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil.
 Vol. 44 nº 4 - July / Aug. of 2011
Vol. 44 nº 4 - July / Aug. of 2011






