Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011
|
ORIGINAL ARTICLE
|
|
Impact of enteral nutrition on acute toxicity and treatment continuity in head and neck cancer patients submitted to intensity-modulated radiotherapy |
|
|
Autho(rs): Liêvin Matos Rebouças1; Elisabeth Callegaro2; Gabriel Oliveira Bernardes Gil3; Maria Letícia Gobo Silva4; Maria Aparecida Conte Maia5; João Victor Salvajoli6 |
|
|
Abstract: INTRODUCTION
Head and neck cancer is the sixth most prevalent tumor in the world and the use of tobacco and alcohol are accountable for 75% of neoplasias in this region(1,2). The role of infection by human papilloma virus seems to be important, mainly in oropharyngeal carcinomas, particularly in young adult individuals(3). The treatment for head and neck cancer consists of surgery, followed or not by radiotherapy (RT) or radical RT, concomitantly or not with chemotherapy. Intensity-modulated radiotherapy (IMRT) has been demonstrating to be advantageous over traditional techniques such as conventional (2D) RT and conformational (3D) RT, as it provides a more homogeneous dose coverage of the target volume, while reducing the dose to adjacent tissues(4,5). The increase in dose is related to an improvement in the tumor management and to a better survival rate. In this scenario, IMRT also plays an important role in improving quality of life, as it is capable of preserving the function of some organs adjacent to the target volume, for example, the salivary glands(6). Malnutrition is a frequent outcome in head and neck cancer patients, not only by the tumor itself, but also as a result of the treatment which can commonly cause side effects such as dysgeusia, dysphagia, xerostomia and mucositis(7,8). In spite of the clear dosimetric gain which can be translated into late toxicity reduction, patients undergoing IMRT may present important acute toxicity during or immediately after the treatment completion, which may adversely impact on nutritional status maintenance and cause interruptions of the treatment. The radiation treatment interruption due to toxicity is one of the effects that should be avoided, as it extends the total treatment time and, as a result, causes a decrease in local control(9). Reports in the literature correlate treatment interruption for only one day with a decrease of 1.4% in local control, and interruption for one week, with a decrease in local control by 10% to 12%(10). Enteral nutritional therapy during RT may minimize the impact of adverse side effects, such as weight loss and treatment interruptions. The most commonly used forms of enteral nutritional therapy are percutaneous endoscopic gastrostomy and nasoenteric feeding tube(11,12). In the present study, the weight loss of patients submitted to IMRT was quantified and correlated with the need of radiotherapy replanning and/or interruption. Additionally, we evaluated the impact of the pre-radiation introduction of nutritional support techniques such as endoscopic percutaneous gastrostomy or nasoenteric feeding tube on body weight and total treatment time in head and neck cancer patients. MATERIALS AND METHODS Population and inclusion criteria Records of all the head and neck cancer patients submitted to IMRT at Hospital A.C. Camargo, São Paulo, Brazil, between January of 2005 and October of 2008 were retrospectively evaluated. Patients submitted to definite radical or adjuvant RT, in association or not with chemotherapy, were included. As an inclusion criterion, it was established that, besides the primary lesion or tumor bed, IMRT would have as a target volume either unilateral or bilateral cervical lymph nodes regions. Thus, we avoided the inclusion of cases in which minimal adverse side effects related to IMRT were expected because of the reduced target volume, which could, consequently, lead to an additional confusion factor for the interpretation of results. Among the 123 patients, 40 were excluded due to: focal irradiation only (14), uncompleted radiation treatment for other causes than the associated toxicity (2), previous history of RT in the tumor region (10) and absence of data about the body weight (14). Therefore, 83 patients were included in the study. The study was submitted to the Committee for Ethics in Research of the institution, and approved under No. 1164/08. Treatment description Preceding the RT, all the patients were submitted to nutritional evaluation and were informed of the possible RT toxicity. They were offered the option of being submitted to enteral nutritional therapy, either by nasoenteral tube or by endoscopic percutaneous gastrostomy. The simulation was performed with the patient in supine position and thermoplastic masks were utilized for immobilization. The patients were then submitted to computed tomography (CT scan) for the radiotherapy planning. The IMRT field arrangement consisted of seven coplanar beams, with no field match line, covering the entire treatment volume, including the supraclavicular fossae when they were part of the target volume. During the course of RT, the patients had their weight measured on a daily basis, and were submitted to a weekly review with the radiation oncologist for assessment ofthe occurrence of morbidity related to the treatment, particularly dysphagia, odynophagia, xerostomia and mucositis. The toxicity was classified according to the acute morbidity criteria of the Radiation Therapy Oncology Group(13) and grouped into grades < 2, and grades > 2. In this same classification system, weight loss is given grade 1 for loss of up to 5% of initial weight, grade 2, between 5% and 15%, and grade 3, the loss greater than 15%(13). At least one CT scan was performed during the course of RT, around the 15th treatment fraction, when the changes in treatment volumes and necessity of replanning would be evaluated. Statistical analysis Descriptive analysis was utilized to summarize the patients’ characteristics, site of the primary tumor, performed treatment (surgery, neo- or adjuvant chemotherapy, and enteral nutritional therapy), and toxicity. The correlation between RT interruption and the variables weight loss and pre-RT enteral nutritional therapy was evaluated by the Fisher’s exact test, while the continuous variables were compared by the Student’s t test, and its correlation was assessed by the Pearson’s correlation. RESULTS Patients’ characteristics The median age was 58.6 years, with a predominance of male individuals (68.7%) and the oropharynx being the most frequent primary tumor site. Most patients presented locally advanced tumors, T3 or T4, and 67% presented positive lymph nodes (N1-N3) (Table 1). 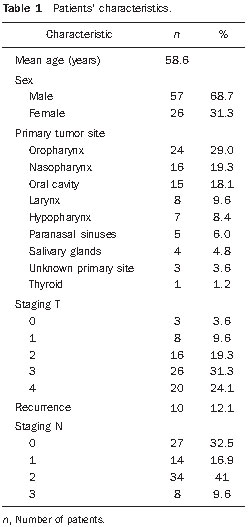 Treatment characteristics In 52 (63%) patients, IMRT was indicated as an adjuvant after surgical resection, and in 31 (37%) the RT was definitive. Radiotherapy with concomitant chemotherapy was performed in 45 (55%) patients, and the other 38 (45%) patients received RT alone. All the patients in the study underwent irradiation of the cervical chain, bilaterally in 69 (83%) patients and unilaterally in 14 (17%) patients. The median final radiation dose was 66 Gy, ranging between 50 and 72 Gy. The RT had a mean duration of 49 consecutive days, ranging between 22 and 66 days. Seventy-eight patients (94%) did not experience any interruption in the course of RT. In the cases of the five patients who experienced it, the interruptions ranged from 4 to 18 days, but in only one case the interruption was longer than 9 days, achieving 18 days. Replanning due to changes in volume was required for 19 patients, 17 of them requiring replanning only once, and two twice. In only 16 patients (19%) enteral nutritional therapy was performed before RT beginning, i.e., with a prophylactic intent. Among these patients, 14 (87.5%) underwent percutaneous gastrostomy and only 2 (12.5%) underwent nasoenteral intubation. Toxicity The presence of significant toxicity was frequently observed during treatment. The number of patients with mucositis, dysphagia or odynophagia and xerostomia > grade 2 at any moment during the course of RT corresponded, respectively, to 54 (64%), 21 (25%) and 34 (41%). Weight loss > 5% of the pre-RT body weight was observed in 58 patients, of which 18 (26.4%) required treatment replanning. Among the 25 patients who did not present weight loss > 5%, only one (0.25%) required replanning (p = 0.009; Fisher’s exact test). Enteral nutritional therapy In spite of the expected positive relationship between the use of enteral nutritional therapy and a less frequent occurrence of weight loss > 5% (Table 2), it was not possible to establish a significant correlation between utilization of enteral nutritional therapy and a lower replanning occurrence. Among the 16 patients who received enteral nutritional therapy, 9 (56.3%) did not require replanning, while among the 67 who did not receive prophylactic enteral nutritional therapy, 55 (82%) did not require replanning (p = 0.741; Fisher’s exact test). 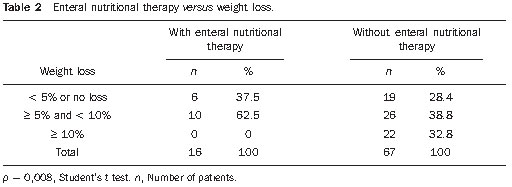 The utilization of enteral nutritional therapy did not demonstrate any significant correlation with the occurrence of RT interruption, nor with its duration (Table 3). 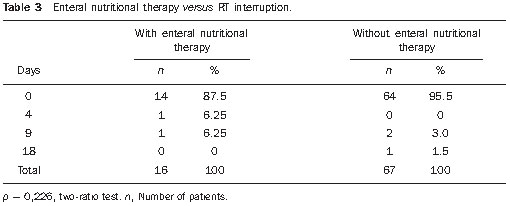 DISCUSSION Patients with advanced head and neck cancer require concomitant RT and chemotherapy. Cisplatin and cetuximab are the drugs most frequently utilized as systemic therapy for head and neck tumors(14). In the present study, 55% of the patients underwent concomitant chemotherapy and RT, and 10% underwent pre-RT induction chemotherapy. Combined RT/chemotherapy in patients with head and neck tumors is associated with a considerable incidence of grade 3 acute toxicity. A high degree of acute mucositis impairs a proper dietary intake. Thus, enteral nutritional therapy becomes an important tool for such patients. The analysis demonstrates that enteral nutritional therapy plays a relevant role in the maintenance of the body weight, as the patients who underwent pre-RT nutrition therapy experienced lesser weight loss as compared with those who didn’t. In the study developed by Corry et al., a statistically significant difference was observed in relation to weight loss among the forms of enteral nutritional therapy. The patients who underwent nasoenteral intubation have averagely lost 3.7 kg, while those who were submitted to percutaneous endoscopic gastrostomy gained 0.8 kg(7). This same study demonstrated that the nasoenteral tube tends to move out of position more easily, requiring repositioning in 62% of cases, while, with percutaneous endoscopic gastrostomy, repositioning was required in only 19% of the patients. However, infections were more prevalent in the group that received percutaneous endoscopic gastrostomy (66%) than in the group that received nasoenteral tube (30%). Other complications resulting from nasoenteral intubation were pharyngeal ulceration, refusal of reintubation, and discomfort caused by the tube, while with percutaneous endoscopic gastrostomy the complications were tube obstruction and colonies grow in the ileum. In the study developed by Scolapio et al., percutaneous endoscopic gastrostomy is recommended as the enteral nutritional therapy when it extends for more than four weeks, considering that, after that period, the presence of a nasoenteral tube is related to laryngeal irritation, gastroesophageal reflux, necrosis and sinusitis(15). In the present study, the possible differences between nasoenteral intubation and percutaneous endoscopic gastrostomy were not evaluated because of the small number of patients submitted to pre-RT enteral nutritional therapy (only 16 cases). Only five (6%) patients required RT interruption, but in four of these cases the interruption was longer than nine days, a fact that tends to reduce the local control, as demonstrated in the study developed by Bese et al., where the interruption of the treatment for a single day caused a decrease of 1.4% in local management, while in one-week interruptions such decrease ranged between 10% and 12%(10). It was not possible to establish a correlation between the need for RT replanning and enteral nutritional therapy. Replanning is required in cases where the patient presents a significant weight loss, with body volume decrease, or in the presence of a tumor volume decrease, and in such circumstances a new immobilization mask has to be made and subsequently a new treatment plan has to be established. In the present study, it was expected that patients who had not been submitted to enteral nutritional therapy and, therefore, presented a greater weight loss, would require a higher number of replannings. Such a correlation was not present, possibly because of the small size of the sample, and also for the fact that only the patients who received prophylactic (i.e. pre-RT) enteral nutritional therapy were considered, and not those who received on a reactive basis, during RT. Besides mucositis, other symptoms arising from the treatment may considerably affect the patients’ nutritional status. Xerostomia, dysphagia and dysgeusia/ageusia have an influence on the appetite loss and, consequently, on the weight loss(16). Intensity-modulated radiotherapy may play a significant role in the minimization of at least two of such symptoms. One of the great advantages of IMRT is the reduction of the rates of late xerostomia because of the reduction of the dose on the salivary glands, particularly the parotid glands. In the present study, 41% of the patients presented xerostomia > grade 2 during RT, a finding similar to the one observed by Chao et al.(17), who have also demonstrated that, although most patients presented dry mouth as a symptom during RT, the salivary glands presented good recovery of their function. In the present study, only patients submitted to IMRT technique were analyzed, therefore no comparison was made with the toxicity associated with conventional RT. The study developed by Kam et al.(18) shows that patients submitted to IMRT presented xerostomia grade 2 to 4, with a much lower toxicity as compared with patients who underwent conventional RT (46.4% versus 85.7%, respectively; p = 0.002), a fact that demonstrates the relevance of IMRT in the reduction of late toxicity. Dysphagia is a frequent symptom in patients with head and neck cancer. Changes in deglutition caused by the presence of the tumor itself are expected(19). Additionally, RT becomes a further factor for the occurrence and severity of dysphagia. Einsbruch et al. have demonstrated that an important mechanism would be the injury of the pharyngeal constrictor muscles additionally to the glottic and supraglottic segments of the larynx in patients undergoing radio-chemotherapy(20). In another study, Gokhale et al. have shown a direct relationship between a higher radiation dose to the pharyngeal constrictor muscles and the need to extend the use of enteral nutritional therapy(21). Reports of up to 20% of long-term dependence on enteral nutritional therapy are found in the literature. Therefore, a reasonable strategy would be the planning of IMRT striving to achieve a lower radiation dose to structures related to deglutition, thus reducing the incidence and severity of dysphagia, which may lead to a lower dependence on enteral nutritional therapy. There are recent reports in the literature suggesting that a reactive approach with enteral nutritional therapy provides excellent results with respect to morbidity, dependence on enteral nutritional therapy and adherence to the treatment. Additionally, prophylactic percutaneous endoscopic gastrostomy, in spite of the lesser weight loss, leads to a higher risk for late esophageal stenosis(22). These factors bring into question the tendency of systematically performing prophylactic enteral nutritional therapy in patients who will be submitted to IMRT for head and neck tumors. However, further studies with more careful analyses of quality of life are necessary to identify those patients who might actually benefit from such an approach. CONCLUSION Body weight loss > 5% was related to a greater probability of RT replanning. In spite of the noticeable benefits of percutaneous endoscopic gastrostomy or nasoenteral intubation in the maintenance of body weight, no gain was observed with prophylactic enteral nutritional therapy in relation to RT interruption or replanning. Considering the currently available medical evidences, we could not objectively recommend the moment during RT course to institute enteral nutritional nor the most appropriate technique to be performed. The decision making shall be done on an individual basis at each institution, and adapted to the needs of each patient. REFERENCES 1. Parkinuyt DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. 2. Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. 3. Psyrri A, Gouveris P, Vermorken JB. Human papillomavirus-related head and neck tumors: clinical and research implication. Curr Opin Oncol. 2009;21:201–5. 4. Duprez F, Madani I, Bonte K, et al. Intensity-modulated radiotherapy for recurrent and second primary head and neck cancer in previously irradiated territory. Radiother Oncol. 2009;93:563–9. 5. Salvajoli JV, Souhami L, Faria SL. Radioterapia em oncologia. Rio de Janeiro, RJ: Medsi; 1999. 6. Fang FM, Chien CY, Tsaai WL, et al. Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy – a longitudinal study. Int J Radiat Oncol Biol Phys. 2008;72:356–64. 7. Corry J, Poon W, McPhee N, et al. Prospective study of percutaneous endoscopic gastrostomy tubes versus nasogastric tubes for enteral feeding in patients with head and neck cancer undergoing (chemo) radiation. Head Neck. 2009;31:867–76. 8. Meuric J, Garabige V, Blanc-Vincent MP, et al. Bonnes pratiques pour la prise en charge ditétique des patients atteints de cancer des voies aérodigestives supérieures. Bull Cancer. 1999;86:843–54. 9. Tyldesley S, Sheehan F, Munk P, et al. The use of radiologically placed gastrostomy tubes in head and neck cancer patients receiving radiotherapy. Int J Radiat Oncol Biol Phys. 1996;36:1205–9. 10. Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68:654–61. 11. van Bokhorst-de van der Schueren MA. Nutritional support strategies for malnourished cancer patients. Eur J Oncol Nurs. 2005;9 Suppl 2:S74–83. 12. Dias MCG, Nadalin W, Baxter YC, et al. Acompanhamento nutricional de pacientes em radioterapia. Rev Hosp Clin Fac Med Univ São Paulo. 1996;51:53–9. 13. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–6. 14. Koukourakis MI, Tsoutsou PG, Karpouzis A, et al. Radiochemotherapy with cetuximab, cisplatin, and amifostine for locally advanced head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2010;77:9–15. 15. Scolapio JS, Spangler PR, Romano MM, et al. Prophylactic placement of gastrostomy feeding tubes before radiotherapy in patients with head and neck cancer: is it worthwhile? J Clin Gastroenterol. 2001;33:215–7. 16. Di Liberto C, Caroprese M, Pizzo G, et al. Oral complications in patients with head and neck cancer after radio-chemotherapy. Mucositis and xerostomia. Recenti Prog Med. 2007;98:302–14. 17. Chao KS, Majhail N, Huang CJ, et al. Intensity modulated radiation therapy reduces late salivary toxicity without compromising tumor control in patients with oropharyngeal carcinoma: a comparison with conventional techniques. Radiother Oncol. 2001;61:275–80. 18. Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–9. 19. Nguyen NP, Smith HJ, Sallah S. Evaluation and management of swallowing dysfunction following chemoradiation for head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2007;15:130–3. 20. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–39. 21. Gokhale AS, McLaughlin BT, Flickinger JC, et al. Clinical and dosimetric factors associated with a prolonged feeding tube requirement in patients treated with chemoradiotherapy (CRT) for head and neck cancers. Ann Oncol. 2010;21:145–51. 22. Clavel S, Fortin B, Desprs P, et al. Enteral feeding during chemoradiotherapy for advanced head-and-neck cancer: a single-institution experience using a reactive approach. Int J Radiat Oncol Biol Phys. 2010 May 24. [Epub ahead of print]. 1. MD, Radiation Oncologist, Instituto do Câncer do Ceará, Fortaleza, CE, Brazil. 2. Medical Student, Faculdade de Ciências Médicas da Santa Casa de São Paulo, Student of Scientific Initiation at Fundação Antônio Prudente, Hospital A. C. Camargo, São Paulo, SP, Brazil. 3. MD, Radiation Oncology Resident, Hospital A. C. Camargo, São Paulo, SP, Brazil. 4. Master, MD, Radiation Oncologist, Department of Radiation Oncology, Hospital A. C. Camargo, São Paulo, SP, Brazil. 5. Master, MD, Head of the Department of Radiation Oncology, Hospital A. C. Camargo, São Paulo, SP, Brazil. 6. PhD, MD, Radiation Oncologist, Instituto do Câncer do Estado de São Paulo (Icesp), São Paulo, SP, Brazil. Mailing Address: Dr. Liêvin Matos Rebouças Rua Doutor José Lourenço, 1154, ap. 202, Aldeota Fortaleza, CE, Brazil, 60115-281 E-mail: lievinr@gmail.com Received July 19, 2010. Accepted after revision November 25, 2010. * Study developed at Hospital A. C. Camargo, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554