INTRODUCTION
Paracoccidioidomycosis (PCM) or South American blastomycosis is a systemic mycosis whose natural progression leads to death in case specific therapy is not instituted(1). The incidence of such disease is restricted to the American continent, occurring from Mexico to Argentina.
The highest incidence rates have been recorded in Brazil, Argentina, Colombia and Venezuela(2). In Brazil, the disease is more frequent in the Southern, Southeastern and Center-Western regions(3). It predominantly affects male individuals in the age range between 30 and 50 years, and is rarely observed in individuals under the age of 14(4). Paracoccidioidomycosis is endemic in the populations living in rural areas, where the incidence is estimated to be between 1 and 3 cases per 100,000 inhabitants(5).
Among the deep mycosis, PCM poses the greatest impact on public health. Affected individuals are in the most active phase of their lives, and the sequels caused by such mycosis are common causes of incapacitation for work. Spontaneous regression of lesions has been very rarely reported(6).
Even though chest high-resolution computed tomography (HRCT) is a clearly superior method both for the analysis of the pattern and distribution of pulmonary findings, and for the assessment of complications (such as excavations and development of fibrosis), plain chest radiography is still the imaging method with the best cost/benefit ratio for the detection of the disease and follow-up of patients(7).
Different systemic antifungal drugs such as itraconazole, a first-generation Triazole derivative can be utilized in the treatment of PCM. Recently, new triazole derivatives were developed; among them, voriconazole, characterized by a wide antifungal spectrum, including invasive and endemic mycotic agents, such as
Paracoccidioides brasiliensis that is the etiological agent of PCM(8).
The objectives of the present analysis were the following:
1 – To review the pulmonary radiographic findings along the progression of chronic PCM, before, during and after treatment, with focus on the patterns, distribution and profusion, by utilizing defined, objective, and reproducible criteria, through the adaptation and simplification of the method already established for analysis of another diffuse pulmonary disease – the ILO (International Labour Office) classification system of chest radiographies of patients with pneumoconioses(9).
2 – To evaluate the differences in the outcomes following treatment with a new triazole antifungal agent, voriconazole, and with the current drug of choice for the treatment of chronic PCM, itraconazole, from the standpoint of radiographic progression of the pulmonary lesions.
MATERIALS AND METHODS
The radiologic analysis was a part of a global comparative and randomized study involving the therapeutics of chronic PCM, prospectively and simultaneously developed in three Brazilian reference centers. Postero-anterior chest radiographies of 39 consecutive patients with chronic PCM with pulmonary involvement, obtained before, during and after antifungal treatment, were analyzed. The patients were divided into two groups as follows: one receiving treatment with voriconazole and the other with itraconazole, randomized, respectively in a 2:1 ratio.
Both female and male patients above 18 years of age were included in the present study. Female patients should not get pregnant during the study because of Triazole derivatives contraindications during gestation. The diagnoses were microbiologically documented by the finding of
Paracoccidioidomicosis brasiliensis in several tissues and/or biological material by means of direct mycological examination, histopathology and/or culture. The study was developed in compliance with the standards of good clinical research practice, and all the participants signed a term of free and informed consent.
Five cases were excluded from the final analysis as four of them did not complete the minimum treatment time and one required a longer treatment because of a concomitant involvement of the central nervous system.
Definition of the study groups
Each patient was identified by a numerical code and, after randomization, started receiving one of the two drugs. Among the 34 patients that completed the study, 21 were treated with voriconazole and 13 with itraconazole (control drug). In case the global clinical response at the end of a six-month period was not satisfactory, the treatment could be extended for up to six additional months at the researcher discretion.
Radiographic analysis – adaptation and simplification of the ILO system
The radiographic images were acquired at the moment of diagnosis and the randomization (basal), one month, two months and three months after starting the treatment, at the end of the treatment (final), and two months after the treatment completion (follow-up). The analysis of the images was independently performed by two radiologists, with eventual disagreements being resolved by consensus. In order for the evaluations to become as reproducible and objective as possible, an adaptation of the ILO pneumoconioses classification system developed 1980(9) was utilized, considering five analysis parameters as follows: 1) small rounded opacities; 2) small irregular opacities; 3) profusion of small opacities; 4) large opacities; 5) lesions extension.
Small rounded opacities are defined as well-delimited nodular images, with diameter > 10 mm, as shown on the pre-treatment chest radiographs of two patients in the study (Figures 1 and 2).
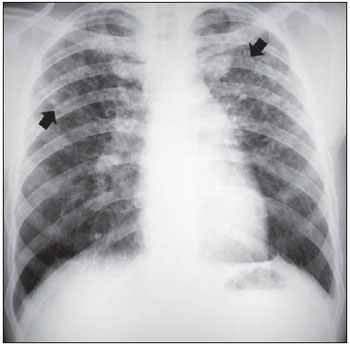
Figure 1. Small rounded opacities (arrows).
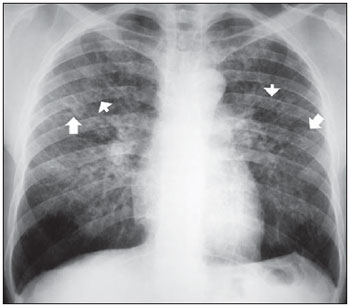
Figure 2. Small irregular opacities (arrows) and small rounded opacities (arrowheads).
Small irregular opacities correspond to linear, reticular or reticulonodular lesions. In the present study, linear, reticular or reticulonodular images whose diameters were < 10 mm were classified as small opacities (Figure 2).
Profusion of alterations (number of small rounded or irregular opacities by area unit or lung zone), was assessed according to four categories:
0, absent small opacities; category
I, small opacities definitely present, although in small number, not affecting the visualization of normal vascular images;
II, numerous small opacities partially obscuring normal vascular images; and
III, quite numerous small opacities, totally impairing the visualization of normal vascular images. For the purpose of statistical analysis, a scale of ordinal values ranging from 0 to 3, corresponding to greater or smaller profusion of lesions was utilized (Table 1).
The terms “large opacities” was employed to describe lesions with > 10 mm and alveolar pattern (Figure 3), being subdivided into four categories as follows: category
O, absence of large opacities; category
A, opacity whose largest diameter is between 1 cm and 5 cm or several opacities with individual diameter > 1 cm, and whose sum of the largest diameters is not > 5 cm; category
B comprises one or more opacities larger and more numerous than those of category
A, whose combined area does not exceed the right upper lung zone; category
C comprises one or more opacities, whose combined area is larger than the upper right lung zone. A scale of ordinal numeral values ranging from 0 to 3 was also utilized for grading large opacities (Table 2).
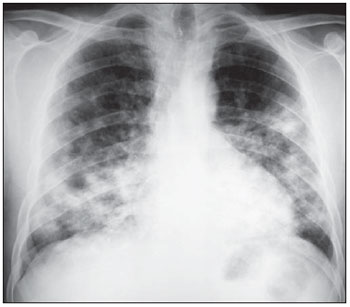
Figure 3. Large opacities in the two lower thirds of the lungs.
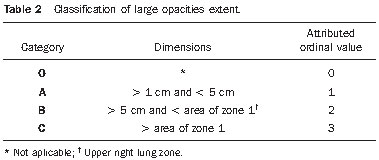
In order to assess the extent and site of lesions, each lung was divided into three zones: upper, middle and lower, by horizontal lines that divide the vertical distance from the pulmonary apex to the hemidiaphragm into three equal parts. The lung zones were denominated as follows: upper right = zone 1, middle right = zone 2, lower right = zone 3, upper left = zone 4, middle left = zone 5 and inferior left = zone 6 (Figure 4).
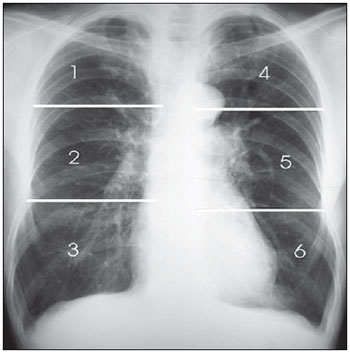
Figure 4. Division of the lungs into six zones.
In each one of the six zones, at each one of the six different times, an ordinal value ranging from zero to a maximum of six was attributed according to the profusion of small opacities and the presence/extent of large opacities. Thus, if for example, the lower lung zones of a given patient (Figure 5) presented small opacities in such a profusion that completely obscured the normal vascular images, and large opacities whose extension was > 5 cm, although not exceeding the upper right lung zone, the ordinal value attributed for each one of these fields would be 5 (category
III =3 + category
B = 2). Also on Figure 5, only small opacities are observed in the middle lung zones, partially impairing the visualization of normal vascular images, each one of these zones are given the value of 2 (category
II = 2).
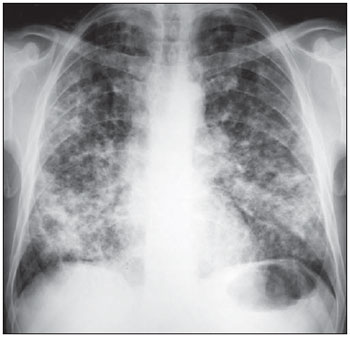
Figure 5. Bilateral and symmetrical, diffuse lung lesions predominating in the middle and lower lung zones.
At each of the six different times, the values of the of the six lung zones were summed up, obtaining a value in ordinal scale (comprised between the minimum of 0 and maximum of 36) that quantifies the pulmonary findings observed on postero-anterior chest radiograph of each of the patients at a given moment along the study.
Statistical analysis
In comparisons between independent samples (different treatments) the hypothesis of equal outcomes was tested. The non-parametric Mann-Whitney test was adopted for that purpose.
In the comparison between paired samples (different times of analysis of radiographs of a same patient), the hypothesis of equal outcomes was tested at two moments by means of the non-parametric Wilcoxon test.
In all of the cases, the significance level of 5% (
p < 0.05) was observed, and the option for non-parametric tests was made because the results were not directly evaluated, but by means of a scale(10).
The variables were defined according to the moment when the radiographic image was acquired, the lung zones and the pulmonary lesion.
RESULTS
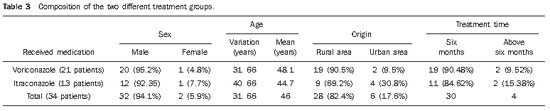
Most of the patients were men (94.1%), and their other characteristics are summarized on Table 3.
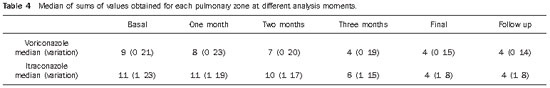
Table 4 demonstrates, for the two patient groups, the medians and the variations (maximum and minimum values) of the sums of values obtained for each lung zone, at each analysis moment.
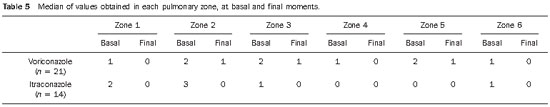
The scores representative of profusion of pulmonary lesions obtained for each one of the six zones, at the basal and final moments, ranged from 0 to 5, and the median values are shown on Table 5.
Comparison between treatments
According to the clinical, laboratory and radiological follow-up, six months were required for the treatment of the patients in both groups, a fact that is in agreement with findings reported by other authors(11). From the standpoint of the radiographic images analysis, the sufficiency of the six months of treatment became evident by means of the fact that no significant alteration was observed on the radiographs at the end of the treatment and in the follow-up two months after the treatment completion (Table 4).
The mean ± a standard deviation validate the hypothesis of equal outcomes in both treatments (
p > 0.05) and demonstrate that at the beginning of the study, the two groups were homogeneous in relation to the radiographic findings, considering both the lung as a whole, as well as the lung zones individually (
p > 0.05).
At the end of the treatment, the radiographic improvement at each lung zone was also similar in both groups (
p > 0.05).
Comparison between moments of analysis
In both groups there was a gradual regression of the pulmonary radiographic alterations along the entire treatment period (
p < 0.05), as exemplified on Figures 6 and 7.
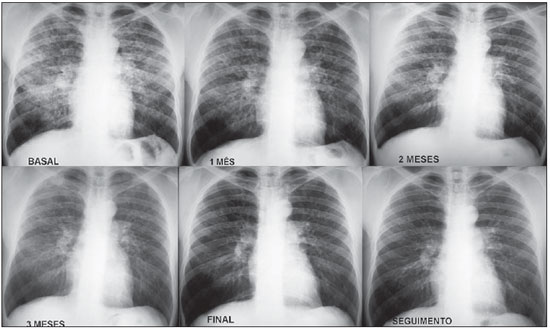
Figure 6. Continuous and gradual improvement of pulmonary radiographic changes in a patient treated with voriconazole.
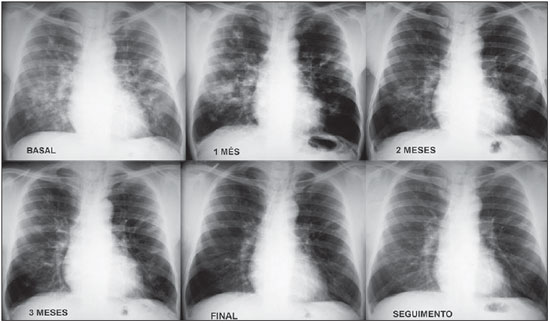
Figure 7. Continuous and gradual improvement in pulmonary radiographic changes in a patient treated with itraconazole.
At the beginning of the treatment, in both groups, a significant difference was observed in the profusion of lesions between upper and middle lung zones (
p < 0,05), with no significant difference between the profusion of lesions in middle and lower lung zones.
At the basal moment, in the group treated with itraconazole, a significant difference was observed in the profusion of lesions in upper
versus lower lung zones (
p < 0.05), and it was possible to infer that there is a symmetrical distribution of the lesions, with no significant difference of results in the comparisons between upper, middle and lower right and left lung zones (
p > 0.05). Such bilateral and symmetrical distribution, with predominance in the middle and lower lung zones, can be observed on Figures 3 and 5.
Additionally, it was observed that there was a tendency of the differences between profusion of lesions at the various lung zones to remain the same after the treatment completion.
Progression of small and large opacities
At the basal moment, radiographic findings in 67.6% of the patients were exclusively small opacities (69.2% of the patients treated with itraconazole and 66.7% of those who received voriconazole), 29.4% presented both small and large opacities (30.8% of the itraconazole group and 28.6% of those who received voriconazole), and in only one case (2.9%) no alteration was noticeable at radiography.
At the end of the treatment, partial regression of the small opacities was observed in 27 patients (79.4%), while in six patients, the lesions remained stable since the beginning of the analysis. Complete regression of large opacities was observed in nine of the ten cases that presented such opacities at the basal moment, six in the group treated with voriconazole and four in the group treated with itraconazole (resolution of large opacities in 90% of the cases). Regression of small and large opacities can be observed on Table 6 and on Figure 8.
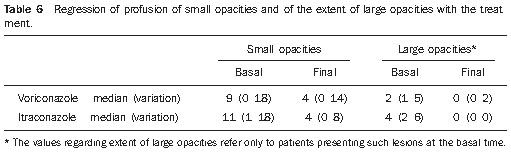
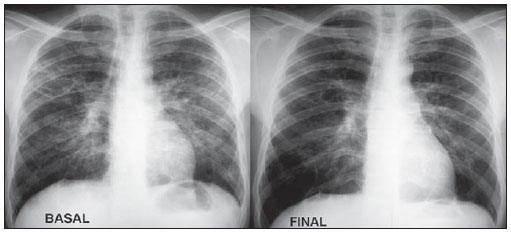
Figure 8. Regression of small opacities with treatment, with irregular striae in the middle lung fields, probably as a result from a cicatricial process.
The nomenclature utilized in the radiological classification proposed for pulmonary findings observed in chronic PCM is highly variable, and a universal consensus on such classification is still to be achieved. The classification range from micronodular, infiltrative and striated lesions(12), productive, productive-exudative and productive-exudative-excavated forms(13), up to infiltrative, nodular and fibrotic patterns(14). Thus, aiming at an objective and reproducible qualification and quantification of these findings, the option was made to classify them according to the adapted ILO classification system, into small and large opacities.
The bilateral and symmetrical distribution of the lesions, with predominance in the middle and lower lung zones, confirmed the findings reported by previous studies(12,15,16). Other authors have also found bilateral lesions, but with a clear predominance in the middle lung zones or perihilar regions(17–19).
The low frequency of the pattern known as “butterfly wings”, characterized by bilateral alveolar opacities in the middle lung zones was confirmed. None of the 34 patients presented exclusively alveolar opacities in the middle lung zones. However, a similar pattern corresponding to large opacities in the middle and also in other lung zones was observed in two patients (5.88%). Cruz et al. have denominated this pattern as pseudopneumonic finding, observing it in 0.5% of their cases(20). Such pattern was found in 10% of the cases followed-up by Martins et al.(21), in 16.6% by Valle et al.(22) and in 2.7% by Machado Filho & Miranda(12).
In the study developed by Funari et al. evaluating pulmonary abnormalities with HRCT, bilateral and symmetrical distribution was observed in the different lung zones, with a tendency to spare the anterior regions of the lower lung zones(7), which, in a certain way, coincides with the “inverted Y” sign on lateral chest radiographs described by Quagliato Jr., where the lingula, the middle lobe and posterior regions of lower and upper lobes are preferentially affected(23). A more recent study also demonstrates distribution of findings in the peripheral and posterior regions of the lungs with a subtle predominance of the middle lung zones(24). Such tendency to spare the anterior regions of the lower lung zones was not evaluated in the present study, as the data obtained were based on postero-anterior radiographic images.
The basic radiographic patterns already described are the following: nodular lesions, reticular lesions and alveolar condensation or opacities, and their possible combinations.
In our study, the nodules were classified as small rounded opacities, with a diameter < 10 mm, and the differences in dimensions among the nodules were not evaluated. On the other hand, larger nodular opacities were classified as large opacities.
Nodule has already been defined as a fundamental radiological lesion in PCM with pulmonary involvement, occurring with various dimensions, from miliary to macronodular, in a same patient(25). Cruz et al. have confirmed the variability of nodules dimensions, referring to their macronodular or pseudotumor forms as blastomycomas(20). According to Valle et al., while the nodules would have diameters between 4 mm and 5 mm, micronodules would have diameters between 1 mm and 2 mm(22). A dissenting opinion was proposed in a previous study, where only micronodular forms(12) were described. With the utilization of HRCT, the variability in nodule dimensions was confirmed, many times with irregular contours(7,24).
It is difficult to separate purely reticular from reticulonodular forms at plain chest radiography. For this reason, the option was made to classify them also as small opacities, whose smallest diameters are > 10 mm. The already proposed classification does not define whether such alterations belong to the group of infiltrative lesions that may resolve with the treatment, or would already correspond to residual interstitial fibrogenic alterations. In this sense, the observation of clinical manifestations might be of some help, as the patients with alveolar/interstitial non-fibrogenic infiltrates generally present mucosal lesions, besides odynophagia and/or dysphagia, while patients with pulmonary fibrogenic alterations present lesions in the dermis and dyspnea(26).
In chronic PCM, cicatricial lesions do not cause large retractable deformities like those occurring in tuberculosis(17), and in order to define its presence a radiographic follow-up of patients is required along their treatment.
Alveolar condensation and opacities, whether nodular or not, > than 10 mm, were classified as large opacities. It has been already suggested that alveolar opacities result from the coalescence of reticular or reticulonodular infiltrates(18). The anatomopathological substrate of the alveolar opacities would be a pneumonic reaction, where acute alveolitis with multiple fungi between the inflammatory cells or within the histiocytes is observed(27). In approximately 10% of cases, such acute alveolitis may surround areas of interstitial inflammation, resulting in the pattern denominated “reversed halo sign” observed at HRCT(28).
In the present study, small opacities would correspond to the interstitial pattern, and the large ones, to the alveolar pattern. In the two treatment groups, approximately two thirds of the patients presented the interstitial pattern, only with small opacities at chest radiographs, and approximately one third of them presented the mixed pattern with alterations belonging both to the group of small and large opacities. Cases with exclusively large opacities (purely alveolar pattern) were not observed, and in only one of the patients the chest radiographic images were always considered as normal. Maybe, HRCT would show changes in such patient with normal radiographic images, although a previous study utilizing HRCT has found a small rate (7%) of patients with chronic PCM, with no pulmonary parenchymal abnormality(7). Most of the previous studies have also demonstrated a predominance of the interstitial or mixed patterns, as compared with the alveolar pattern(12,22).
In agreement with most of the series in which CT or tomograms exams were not utilized, excavated lesions could not be reliably be characterized. Excavations occur in previous condensation foci, and their appropriate detection and analysis require computed tomography studies or linear planigraphy studies(20). In the study developed by Funari et al. with 41 patients, where the imaging method analysis was HRCT, excavated lesions whose diameters ranged between 1 cm and 4.8 cm were found in 17.1% of the cases(7). Other author have found excavations with a frequency of 36%(29,30) and 42.9%(25). At HRCT, excavations with irregular walls and internal septa are also described(31).
Atelectasis, pleural involvement, lymph nodes enlargement or calcifications were not observed. The absence of calcifications is an additional information that aids in the differential diagnosis with tuberculosis, a fact that emphasizes the need to search for associated diseases such as tuberculosis or histoplasmosis, whenever calcifications are present(16). Another differentiation factor with tuberculosis should be highlighted: the rarity of lobar atelectasis, due to the tendency of obstructive lesions not occurring in the primary and lobar bronchi in chronic PCM(12).
A continuous and gradual regression was observed in the profusion of radiographic findings in 27 of the 34 patients. In one case alterations could not be demonstrated, while six cases presented stable lesions at the different moments of analysis. In the case with chest radiographs considered as normal the patient had already received a previous treatment with sulfas and had presented alterations at radiography for five years ago. Two of the cases with stable lesions had also been irregularly treated for PCM, The remaining four cases with stable lesions had not been previously treated, but, before the treatment was initiated, they had already presented signs and symptoms of the disease in periods ranging from four months to four years. The long time with the symptoms and the previous antifungal treatment support the possibility that cicatricial alterations were already present at the basal moment of analysis.
Most of the previous studies have reported a more favorable progression of the radiographic findings at the third month of treatment(32,33).
Small opacities presented partial regression, while the large ones presented complete regression in nine of the ten cases that presented such opacities at the basal moment. Thus, it may be suggested that the resolution of areas of alveolar consolidation and macronodules would be an indication of appropriate response to the treatment.
At the treatment completion, small opacities predominated in the middle and lower lung zones, remaining with bilateral and symmetrical distribution, associated with increase in peripheral hypertransparent areas that might correspond to emphysema(15), bubbles(34) or even to areas of air trapping resulting from the involvement of small air ways.
CONCLUSIONS
1. The information reported by previous studies in the literature regarding patterns, distribution and profusion of lung radiographic findings in chronic PCM are confirmed.
2. No statistically significant difference was observed in the radiographic progression of chronic PCM between patients treated with voriconazole and itraconazole.
3. The utilization of an adaptation of the ILO model of pneumoconioses classification demonstrated to be useful in the objective assessment of the progression of diffuse pulmonary radiographic findings along the treatment of patients with chronic PCM.
Acknowledgements
The authors wish to express their gratitude to the Ambulatory of Infectious and Parasitic Diseases of Hospital de Clínicas da Universidade Federal do Paraná and to their patients, for providing the chest radiographs utilized in the present study.
REFERENCES
1. Montenegro MR, Franco M. Pathology. In: Franco M, Lacaz CS, Restrepo-Moreno A, et al., editors. Paracoccidioidomycosis. Boca Raton: CRC Press; 1994. p. 131–50.
2. Bethlem NM, Lemle A, Bethlem E, et al. Paracoccidioidomycosis. Semin Respir Med. 1991;12:81–97.
3. Wanke B, Londero AT. Epidemiology and paracoccidioidomycosis infection. In: Franco M, Lacaz CS, Restrepo-Moreno A, et al., editors. Paracoccidioidomycosis. Boca Raton: CRC Press; 1999. p. 109–20.
4. Londero AT, Ramos CD. Paracoccidioidomicose. Estudo clínico e micológico de 260 casos observados no interior do Estado do Rio Grande do Sul. J Pneumol. 1990;16:129–32.
5. Restrepo A, Greer DL. Paracoccidioidomycosis. In: DiSalvo AF, editor. Occupational mycoses. Philadelphia: Lea & Febiger; 1983. p. 43–64.
6. Melo IS, Londero AT. Spontaneously resolving pulmonary lesions in paracoccidioidomycosis. Case report and review. Mycopathologia. 1983;82:57–9.
7. Funari M, Kavakama J, Shikanai-Yasuda MA, et al. Chronic pulmonary paracoccidioidomycosis (South American blastomycosis): high resolution CT findings in 41 patients. AJR Am J Roentgenol. 1999;173:59–64.
8. Espinel-Ingroff A. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198–202.
9. International Labour Office. Guidelines for the use of ILO International Classification of Radiographs of Pneumoconiosis. Occupational Safety and Health Series No. 22. Geneva: International Labour Office; 1980. p. 1–48.
10. Glantz SA. Primer of biostatistics. 5th ed. San Francisco: McGraw-Hill; 2002.
11. Naranjo MS, Trujillo M, Munera MI, et al. Treatment of paracoccidioidomycosis with itraconazole. J Med Vet Mycol. 1990;28:67–76.
12. Machado Filho J, Miranda JL. Considerações relativas à blastomicose sul-americana. Da participação pulmonar entre 338 casos consecutivos. O Hospital. 1960;58:23–42.
13. Passos Filho MCR. Blastomicose sul-americana. Comentários em torno de 83 casos de localização pulmonar – classificação radiológica. O Hospital. 1966;70:127–52.
14. Gutiérrez F, Silva M, Peláez F, et al. The radiological appearances of pulmonary paracoccidioidomycosis and the effects of ketoconazole therapy. J Pneumol. 1985;11:1–12.
15. Rodríguez C, Rincón NL, Troconis-García G. Contribution to the study of paracoccidioidomycosis brasiliensis in Venezuela. Considerations on 62 cases studied with special reference to respiratory localizations. Mycopathologia. 1961;15:115–38.
16. Hernandez HS. Clinical and radiological aspects of paracoccidioidomycosis. Proceedings of the First Pan American Symposium on Paracoccidioidomycosis; 1971 Oct; Medellín, Colombia.
17. Gonçalves AP, Bardy C. Aspectos clínicos e radiológicos da blastomicose brasileira pulmonar. O Hospital. 1946;30:213–43.
18. Gutiérrez F. Radiological follow-up in five cases of paracoccidioidomycosis. Proceedings of the First Pan American Symposium on Paracoccidioidomycosis; 1971 Oct; Medellín, Colombia.
19. Magalhães A. Paracoccidioidomicose (blastomicose sul-americana). Aspectos radiológicos. Rev Hosp Clin Fac Med São Paulo. 1980;35:147–53.
20. Cruz MFA, Santos Filho RA, Cardoso RC, et al. Aspectos radiográficos intratorácicos da paracoccidioidomicose. Revisão de 170 casos. Radiol Bras. 1989;22:169–77.
21. Martins S, Gerhardt Filho G, Monteiro DJ. Aspectos clínicos e radiológicos da paracoccidioidomicose. J Bras Med. 1984;46:71–9.
22. Valle ACF, Guimarães RR, Lopes DJ, et al. Aspectos radiológicos torácicos na paracoccidioidomicose. Rev Inst Med Trop São Paulo. 1992;34:107–15.
23. Quagliato Jr R. Considerações a respeito da paracoccidioidomicose e sua correlação com níveis séricos de alfa-1-antitripsina [dissertação de mestrado]. Campinas: Universidade Estadual de Campinas; 1980.
24. Souza ASS Jr, Gasparetto EL, Davaus T, et al. High-resolution CT findings of 77 patients with untreated pulmonary paracoccidioidomycosis. AJR Am J Roentgenol. 2006;187:1248–52.
25. Bardy C. Sinais radiológicos pulmonares da blastomicose sul-americana. J Bras Med. 1962;6:484–8.
26. Restrepo A, Tobón AM, Agudelo CA, et al. Co-existence of integumentary lesions and lung x-ray abnormalities in patients with paracoccidioidomycosis (PCM). Am J Trop Med Hyg. 2008;79:159–63.
27. Tuder RM, el Ibrahim R, Godoy CE, et al. Pathology of the human pulmonary paracoccidioidomycosis. Mycopathologia. 1985;92:179–88.
28. Gasparetto EL, Escuissato DL, Davaus T, et al. Reversed halo sign in pulmonary paracoccidioidomycosis. AJR Am J Roentgenol. 2005;184:1932–4.
29. Kauer CL. Paracoccidioidomicose: tomografia computadorizada de alta resolução das alterações Pleuro-pulmonares [tese de doutorado]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2000.
30. Muniz MAS, Marchiori E, Magnago M, et al. Paracoccidioidomicose pulmonar: aspectos na tomografia computadorizada de alta resolução. Radiol Bras. 2002;35:147–54.
31. Marchiori E, Moraes HP, Muniz MAS, et al. Paracoccidioidomicose: correlação da tomografia computadorizada de alta resolução com a anatomopatologia. Radiol Bras. 2000;33:333–40.
32. Restrepo A, Gómez I, Robledo J, et al. Itraconazole in the treatment of paracoccidioidomycosis: a preliminary report. Rev Infect Dis. 1987;9 Suppl 1:S51–6.
33. Del Negro G. Ketoconazole in paracoccidioidomycosis. A long-term therapy study with prolonged follow-up. Rev Inst Med Trop São Paulo. 1982;24:27–39.
34. Restrepo A, Gómez I, Cano LE, et al. Treatment of paracoccidioidomycosis with ketoconazole: a three-year experience. Am J Med. 1983;74:48–52.
1. Master, MD, Radiologist, Hospital de Clínicas da Universidade Federal do Paraná (UFPR) and Clínica DAPI, Curitiba, PR, Brazil.
2. PhD, Associate Professor, Division of Infectology, Department of Internal Medicine, Clinical Staff Director of Hospital de Clínicas da Universidade Federal do Paraná (UFPR), Curitiba, PR, Brazil.
3. Full Professor of Radiology, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil.
4. Doctor Professor, Head of the Division of Radiology, Department of Medicine, Universidade Federal do Paraná (UFPR), Curitiba, PR, Brazil.
Mailing Address:
Dr. Cristian Saievicz de Moraes
Rua Lamenha Lins, 635, ap. 801, Rebouças
Curitiba, PR, Brazil, 80250-020
E-mail: saievicz@hotmail.com
Received June, 25, 2010.
Accepted after revision November 25, 2010.
* Study developed at the Unit of Radiology, Department of Medical Practice, Hospital de Clínicas da Universidade Federal do Paraná (UFPR), Curitiba, PR, Brazil.
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011