Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011
|
ORIGINAL ARTICLE
|
|
Computed tomography findings of pulmonary tuberculosis in adult AIDS patients |
|
|
Autho(rs): Lanamar Aparecida de Almeida1; Mario Flores Barba2; Fernando Alves Moreira3; Sidney Bombarda4; Sebastião Andr de Felice5; Edenilson Eduardo Calore6 |
|
|
Keywords: Tuberculosis; Tomography; Chest; HIV; AIDS; SIDA. |
|
|
Abstract: INTRODUCTION
Tuberculosis is one of the most common complications associated with infection by the human immunodeficiency virus (HIV) worldwide(1). AIDS promotes progression from latent to active disease in co-infected patients(2). In co-infected individuals, active tuberculosis development index is 10 to 30 times higher than in individuals infected by Mycobacterium tuberculosis alone(3). Chest radiography is the imaging method of choice in the initial evaluation and follow-up of pulmonary tuberculosis(4). However, in 6% to 20% of HIV-positive patients with pulmonary tuberculosis conventional radiography may present as absolutely normal(5). Many studies have described radiological manifestations of tuberculosis(5–11). Lee et al.(12) have investigated the usefulness of computed tomography (CT) in the differentiation between active and inactive tuberculosis. Pereira et al.(13) have described the tomographic findings of primary pulmonary tuberculosis initially manifested as lobar consolidation. In 89 patients with active tuberculosis evaluated with thin-slice CT, the following findings were most frequently observed: centrilobular linear opacities (92%), lobular consolidation (62%), acinar nodule (61%), cavity (36%) and ground glass opacity (35%). In another study developed by Hatipo?lu et al.(14) with thin-slice CT, “tree-in-bud” pattern, nodules of up to 8 mm in diameter and consolidations were frequently found in patients with active disease and in none of the patients with inactive disease. The presence of cavity is a relevant sign of active disease. High-resolution CT (HRCT) demonstrates small cavities intermingled with consolidations that many times are not visible at conventional radiography(4). A study developed by Im et al.(15) has demonstrated that the prevalence of cavities at tomography was 58% (24/41), while, at plain chest radiography, the prevalence was 22% (9/41). According to Leung et al., thick-walled cavities are observed in up to 76% of the patients with pulmonary tuberculosis at the time of the diagnosis(16). Centrilobular nodules with segmental distribution representative of bronchogenic dissemination of tuberculosis are also frequently observed in the active phase of the disease (82% to 100%)(16). Bronchial walls thickening occurs in 62% of the cases and bronchiectasis is observed in 23% of the patients. The “tree-in-bud” pattern is present in up to 57% of cases(17). Comparative studies evaluating tomographic findings of pulmonary tuberculosis in both HIV-positive and HIV-negative patients are not so frequently found in the literature(18–20). As compared with the HIV-negative population, AIDS patients present greater probability of ganglia involvement, bronchial dissemination, miliary disease, extrapulmonary disease, and normal plain chest radiography(21). The radiological findings of tuberculosis and HIV/AIDS co-infection are dependent upon the immune status of the patient(22). In HIV-positive patients with CD4 count < 200 cel/mm3, the radiographic findings are those typically described in the primary presentation of the infection(22), while in HIV-positive patients with CD4 count > 200 cel/mm3, the tomographic findings of tuberculosis are those typically observed in cases of disease reactivation(22). The present study describes the tomographic findings of pulmonary tuberculosis in adult patients admitted to an infectious diseases reference hospital, in an attempt to establish associations between tomographic findings of pulmonary tuberculosis and CD4 count in adult AIDS patients. The study was initiated after approval by the Committee for Ethics in Research of Instituto de Infectologia “Emílio Ribas” (IIER), under Research Protocol No. 47/06 and authorization No. 390/2006. MATERIALS AND METHODS The present study included cases of tuberculosis in HIV-positive adult patients admitted to Instituto de Infectologia “Emílio Ribas” and notified by the Epidemiology Service between January/2003 and November/2006, according to the following inclusion criteria: a) HIV-positive at Western Blot and classified as AIDS (categories C and D, according to CDC – Center for Disease Control and Prevention of the United States of America; the most recent classification was issued in 1993); b) adult inpatients (>18 years of age); c) positive sputum culture for M. tuberculosis; d) patients who underwent chest CT by the moment of the diagnosis, or up to 60 days before or 30 days after starting the treatment. Chest CT The chest CT images included in the present study were requested by the patients’ attending physicians, for diagnosis or follow-up. All the examinations were performed in a Light-Speed multislice eight-channels equipment (General Electric Medical Systems; Milwaukee, WI, USA), manufactured in 2002, with the patient in dorsal decubitus, and intravenous non-ionic iodinated contrast medium injection with an automated contrast infusion system. The image sections were acquired at the end of inspiration, from the apex to the diaphragm, utilizing the standard technique in the majority of the patients and, in some cases, the high resolution technique (HRCT). The tomographic images were reviewed by the authors, and the pulmonary findings patterns were described according to the “Brazilian Consensus on Terminology Used to Describe Computed Tomography of the Chest”(23). Statistical analysis The following data were included in a databank: age distribution, time of HIV virus infection, interval between the disease diagnosis and the CT scan, death, CD4 levels (< 200 cel/mm3 and > 200 cel/mm3), associated diseases, tuberculosis treatment and antiretroviral therapy. The data were fed into a database built with the software SPSS 14.0 for Windows, version 14.0.1 of November 18, 2005, and treated by means of descriptive statistics, with calculation of percentages, means, frequencies and standard deviation. The association between CD4 levels and radiological findings was evaluated by the exact Fisher’s test; considering bilateral tests with statistical significance level of 5% or 0.05 (Table 1). RESULTS Among 300 HIV-positive inpatients with tuberculosis during the study period, only 70 had undergone chest CT, and only 45 of these patients met the above mentioned criteria. Of these 45 patients, 35 presented CD4 count < 200 cel/mm3 [30 of men (85.7%) and 5 women (14.3%)]. The remaining 10 patients presented CD4 count > 200 cel/mm3, with nine (90%) being men and one (10%) women. The mean age of the 45 patients was 38 years and nine months, with a standard deviation of 9.6. Among the cases with CD4 count < 200 cel/mm3, the mean age was 38 years and seven months, with a standard deviation of 9.0, while in the cases with CD4 count > 200 cel/mm3, the mean age was 40years and four months, with standard deviation of 11.9. Surprisingly, none of the patients in the present study had undergone regular treatments for tuberculosis or AIDS. Tomographic findings Table 1 shows the frequency of tomographic findings in decreasing order and respective association with CD4 counts of the 45 cases. Mediastinal and/or hilar lymph node enlargement (Figures 1 and 2) 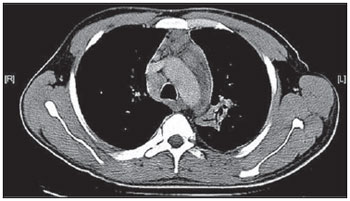 Figure 1. Homogeneous lymph node enlargement – Chest CT. Homogeneous right inferior paratracheal, retrotracheal, and para-aortic, lymph node enlargements, associated with opacity with air bronchogram in a view of the apicoposterior segment of the left upper lobe. 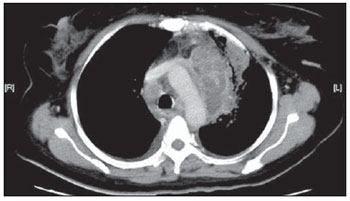 Figure 2. Hypodense lymph node enlargement – Chest CT. Right lower paratracheal, confluent retrotracheal lymph node enlargement, with tomographic signs of necrosis in a view of para-aortic ganglionar chain, associated with small bilateral pleural effusion and upper left pulmonary consolidation. Thirty-one (68.8%) of the patients presented mediastinal and/or hilar lymph node enlargement. Among these patients, 21 (67.7%) presented CD4 count < 200 cel/mm3, while 10 (32.3%) presented CD4 count > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 21 (60%) of the 35 patients presented mediastinal and/ or hilar lymph node enlargement, while 10 (100%) of 10 patients presented such tomographic finding. Pleural effusion Twenty nine (64.4%) patients presented pleural effusion. Of these patients, 23 (79.3%) presented CD4 count < 200 cel/mm3, while 6 (20.7%) presented CD4 count > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 23 (65.7%) of the 35 (60.0%) patients presented pleural effusion and 6 (60%) of the 10 patients presented such tomographic finding. Among the 29 cases with pleural effusion, 20 (68.9%) were bilateral and with small volume. In none of the cases was pleural effusion the sole radiological finding. The CD4 level ranged from 6 cel/mm3 to 540 cel/mm3 (mean = 165 cel/mm3). Centrilobular nodules with segmental distribution (Figure 3) 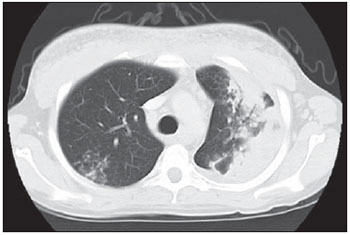 Figure 3. Centrilobular nodules with segmental distribution – Chest CT. Centrilobular nodules with segmental distribution, confluent in the posterior segment of the right upper lobe. Extensive consolidation in the upper left lobe is observed. Twenty-six (57.7%) patients presented centrilobular nodules with segmental distribution. Of these patients, 20 (76.9%) presented CD4 count < 200 cel/mm3, while 6 (23.1%) presented CD4 count > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 20 (57.1%) of the 35 patients presented centrilobular nodules with segmental distribution and 6 (60%) of the 10 patients presented such tomographic finding. It is important to note that, in spite of the absolute number of cases with centrilobular nodules of segmental distribution being smaller in patients with CD4 count > 200 cel/mm3, the percentage of patients with this tomographic finding is higher in this group (60% of the cases). Parenchymal consolidation (Figure 4) 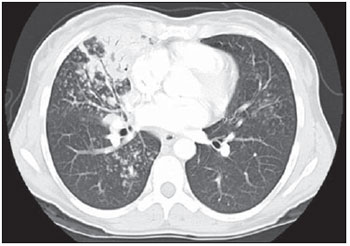 Figure 4. Segmental consolidation in the anterior segment of the right upper lobe – Chest CT. Segmental consolidation in the anterior segment of the right upper lobe caused by the confluence of multiple centrilobular nodules. Notice centrilobular nodules of segmental distribution in the posterior segment of the right inferior lobe and inferior lingular segment of the left lung. Twenty-four (53.3%) patients presented parenchymal consolidation. Of these patients, 15 (62.5%) presented CD4 count < 200 cel/mm3, while 9 (37.5%) presented CD4 count > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 15 (42.5%) of the 35 patients presented parenchymal consolidations, and 9 (90%) of the 10 patients presented such tomographic finding. As regards topographic distribution of the parenchymal consolidations, predominance in the upper lobes was observed. It can be perceived that, although the absolute number of cases with parenchymal consolidation is smaller in patients with CD4 count > 200 cel/mm3, the percentage of patients with this tomographic finding in higher in this group (90% of the cases). Micronodular confluence Seventeen (37.7%) patients presented micronodular confluence. Of these patients, 13 (76.5%) presented CD4 count < 200 cel/mm3 and 4 (23.5%) presented CD4 count > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 13 (37.1%) of the 35 patients presented micronodular confluence, and 4 (40%) of the 10 patients presented such tomographic finding. It can be perceived that, although the absolute number of cases with micronodular confluence is smaller in patients with CD4 count > 200 cel/mm3, the percentage of patients with this tomographic finding in higher in this group (40% of the cases). Ill defined nodules with centrilobular distribution (Figure 5) 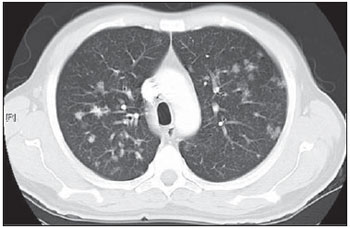 Figure 5. Ill defined nodules with centrilobular distribution – Chest CT. Bilateral, ill defined nodules with centrilobular distribution. Sixteen (35.5%) patients presented ill defined nodules with centrilobular distribution. Of these patients, 12 (75%) presented CD4 count < 200 cel/mm3 and 4 (25%), CD4 > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 12 (34.3%) of the 35 patients presented ill defined nodules with centrilobular distribution, and 4 (40%) of the 10 patients presented such tomographic finding. It can be perceived that, although the absolute number of cases with nodules is lower in patients with CD4 count > 200 cel/mm3, the percentage of patients with this tomographic finding is higher in this group (40% of the cases). “Tree-in-bud” pattern (Figure 6) 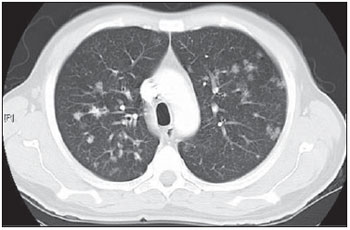 Figure 6. “Tree-in-bud” pattern – Chest CT. Bilateral tubular images in a treelike branching with minute nodulation at the extremities, most noticeable in the right lung, associated with right-sided small pleural effusion. Thirteen (28.9%) of the patients presented the “tree-in-bud” pattern. Of these patients, 9 (69.2%) presented CD4 count < 200 cel/mm3, while 4 (30.8%) presented CD4 count > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 9 (25.7) of the 35 patients presented the “tree-in-bud” pattern, and 4 (40%) of the 10 patients presented such tomographic finding. It can be perceived that, although the absolute number of cases with the “tree-in-bud” pattern is smaller in patients with CD4 count > 200 cel/mm3, the percentage of patients with this tomographic finding is higher in this group (40% of the cases). Bronchial wall thickening Twelve (26.6%) patients presented bronchial wall thickening. Of these patients, 8 (66.72%) presented CD4 count < 200 cel/mm3, while 4 (33.3%) presented with CD4 count > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 8 (22.9%) of the 35 patients presented bronchial wall thickening, and 4 (40%) of the 10 patients presented such tomographic finding. It can be perceived that, although the absolute number of cases with bronchial wall thickening is smaller in patients with CD4 count > 200 cel/mm3, the percentage of patients with this tomographic finding is higher in this group (40% of the cases). Thick-walled cavity (Figure 7) 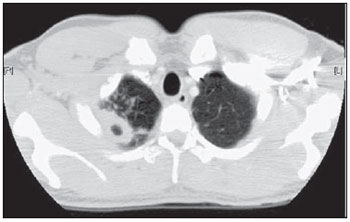 Figure 7. Thick walled cavity in the upper right lobe – Chest CT. Thick walled cavity in the posterior segment of the right upper lobe. Centrilobular nodules also suggestive of disease activity are observed. Ten (22.2%) patients presented thick-walled cavity. Among them, 7 (70%) presented CD4 count < 200 cel/mm3 and 3 (30%), CD4 > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 7 (20%) of the 35 patients presented thick-walled cavity, and 3 (30%) of the 10 patients presented such tomographic finding. The upper right lobe, with four cases (40%), was the most frequent site with this finding. It can be perceived that, although the absolute number of cases with thick walled cavity is smaller in patients with CD4 count > 200 cel/mm3, the percentage of patients with this tomographic finding is higher in this group (30% of the cases). Miliary nodules (randomly distributed small nodules) Nine (20%) patients presented miliary nodules. Of these patients, 8 (88.9%) presented CD4 count < 200 cel/mm3, while 1 (11.1%) presented CD4 > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 8 (22.9%) of the 35 patients presented miliary nodules, and 1 (10%) of the 10 patients presented such tomographic finding. Cylindrical bronchiectasis Six (13.3%) patients presented cylindrical bronchiectasis. Of these patients, four (66.7%) presented CD4 count < 200 cel/mm3 and two (33.3%), CD4 count > 200 cel/mm3. Based on the total number of patients with CD4 count< 200 cel/mm3 (35) and with CD4 count > 200 cel/mm3 (10), the authors observed that, respectively, 4 (11.4%) of the 35 patients presented cylindrical bronchiectasis, and 2 (20%) of the 10 patients presented such tomographic finding. As regards topographic distribution of the cylindrical bronchiectasis, one observed a predominance of this finding in the lower lobes (four cases - 66.6%). It can be perceived that, although the absolute number of cases with cylindrical bronchiectasis is smaller in patients with CD4 count > 200 cel/mm3, the percentage of patients with this tomographic finding is higher in this group (20% of the cases). DISCUSSION According to Castañer et al.(22), the radiological manifestations of tuberculosis in AIDS reflect the level of immunosuppression. Patients with tuberculosis and CD4 count > 200 cel/mm3 present radiological findings similar to those observed in HIV-negative patients. HIV-positive patients with CD4 count < 200 cel/mm3 tend to present radiological findings similar to the ones of primary pulmonary tuberculosis(22). Lymph node enlargement is the radiological hallmark of primary tuberculosis, and its prevalence decreases with age, being reported with a much lower frequency in adults(16). On the other hand, lymph node enlargement is commonly seen in tuberculosis in AIDS patients. More than 60% of the AIDS patients with tuberculosis present hilar and mediastinal lymph node enlargement(22). Laissy et al.(18), in a comparative study approaching the use of HRCT among HIV-positive and HIV-negative patients, have demonstrated that the most frequent abnormality in HIV-positive patients was lymph node enlargement. In HIV-positive patients, lymph node enlargement may present peripheral contrast medium uptake, which is radiologically translated into a hypodense center and peripheral enhancement(21,24). The study developed by Im et al.(15) reports that the hypodense area corresponds to necrosis, and the peripheral enhancement of the lymph node enlargement limits results from the hypervascularization secondary to the inflammatory process. In the present 45-case study, 68.8% of the patients presented mediastinal or mediastinal/hilar lymph node enlargement, while hypodensity in lymph node areas after intravenous contrast injection was observed in seven cases (15%). All these patients presented CD4 count < 200 cel/mm3. Based on such observations, it is possible to infer that this lymph node enlargement pattern is not pathognomonic of tuberculosis, but, the presence of this finding indicates severe immunosuppression. In the study developed by Hulnick et al.(25) with HIV-negative patients, cavities were the parenchymal changes most commonly associated with pleural effusion. In none of the cases was pleural effusion a sole tomographic manifestation. Of the 45 cases in the present study, 64.4% patients presented bilateral and small pleural effusion in association with other tomographic findings, such as parenchymal consolidation, bronchogenic dissemination and mediastinal lymph node enlargement. Centrilobular nodules with segmental distribution representative of bronchogenic dissemination of tuberculosis are the most frequent tomographic findings in the active phase of the disease, and is present in up to 82% of the cases(12). Such finding was the third most frequently observed in the present study (57.7%) but with no statistically significant difference between patients with CD4 count < 200 cel/mm3 or CD4 count > 200 cel/mm3. Im et al.(15), in a study with 41 immunosuppressed patients with pulmonary tuberculosis, have documented that the centrilobular lesion results from bronchogenic dissemination and that this was the most common finding in active disease, occurring in 95% of their patients. In the present study, the authors could observe that the frequency of parenchymal consolidation was significantly higher in patients with CD4 count > 200 cel/mm3. The study developed by Leung et al.(26) suggests that, in HIV-positive patients, atypical opacities with involvement of the middle and lower lobes may be secondary to the bronchogenic dissemination of the disease. According to these authors, atypical opacities were the most frequent finding in the HIV/tuberculosis association, with bronchogenic dissemination in 57% and miliary dissemination, in 17% of patients. According to McGuinness et al.(27), miliary nodules are observed in more than 13% of HIV-positive patients who developed tuberculosis. In the present study, nine cases (20%) presented miliary nodules. Among the 45 cases in the present study, 10 (22.2%) presented with cavities, whose location frequency was slightly greater in the upper right lobe (four cases; 40%). Similarly, the low prevalence of cavities was observed in HIV-positive and negative patients (Leung et al.(26); Laissy et al.(18)). According to Andreu et al.(28), cavities can be either single or multiple and, usually, present thickened and irregular walls, with a small amount of fluid inside with air-fluid level. However, according to Aviram et al.(29), cavitation was not frequent in AIDS, even in severely immunocompromised patients, except in cases of multiple-drug resistance. Among the 45 cases in the present study, six (13,%) presented radiological signs of bronchiectasis, predominantly in the lower lobes. In the study developed by Laissy et al.(18), bronchiectasis was more frequently found in HIV-negative patients than in HIV-positive patients. According to McAdams et al.(30), bronchiectasis is a common complication of endobronchial tuberculosis, associated with bronchostenosis, being radiologically translated, among other signs, into obstructive pneumonia and mucoid impaction. According to Laissy et al.(18) and Gutiérrez et al.(31), atypical manifestations of tuberculosis are more frequently observed in HIV-positive patient than in HIV-negative patients, i.e., lymph node enlargement and pleural effusion are most frequently found in AIDS patients. Such findings were similar to those observed in the present study that also demonstrated agreement with studies developed by Leung et al.(26), which have demonstrated that HIV-positive patients present low prevalence of cavities and high prevalence of lymph node enlargement. CONCLUSION In the present study, by order of frequency, the main findings in tuberculosis in AIDS patients were the following: mediastinal and/or hilar lymph node enlargement, pleural effusion, centrilobular nodules with segmental distribution and consolidation. In this study, differently from reports in the literature, mediastinal/hilar lymph node enlargement and consolidation were significantly more frequent in patients with CD4 count > 200 cel/mm3. It is important to highlight that lymph nodes with hypodense centers were most frequently observed in severely immunosuppressed patients (CD4 count < 200 cel/mm3). Bilateral pleural effusion in tuberculosis in AIDS patients is a frequent finding, differently from what occurs with immunocompetent patients. It is important to reinforce the fact that normal conventional chest radiography does not rule out the diagnosis of tuberculosis, particularly in AIDS patients and, for that reason, CT scans should be performed in all patients with clinical suspicion of this disease. REFERENCES 1. WHO. Global tuberculosis control: surveillance, planning, financing. Geneva, Switzerland: World Health Organization; 2006. [acessado em 4 de janeiro de 2008]. Disponível em: htpp://www. who.int/tb/publications/global_report/en/index.html 2. Batungwanayo J, Taelman H, Dhote R, et al. Pulmonary tuberculosis in Kigali, Rwanda. Impact of human immunodeficiency virus infection in clinical and radiographic presentation. Am Rev Respir Dis. 1992;146:53–6. 3. WHO. Guidelines for HIV: surveillance among tuberculosis patients (Second edition). Geneva, Switzerland; 2004. [acessado em 4 de janeiro de 2008]. Disponível em: http://www.who.int/tb/publications/global_report/en/index.html 4. Bombarda S, Figueiredo CM, Funari MBG, et al. Imagem em tuberculose pulmonar. J Pneumol. 2001;27:329–40. 5. Palmieri F, Girardi E, Pellicelli AM, et al. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002;30:68–74. 6. Krysl J, Korzeniewska-Kosela M, Müller NL, et al. Radiologic features of pulmonary tuberculosis: an assessment of 188 cases. Can Assoc Radiol J. 1994;45:101–7. 7. Khan MA, Kovnat DM, Bachus B, et al. Clinical and roentgenographic spectrum of pulmonary tuberculosis in the adult. Am J Med. 1977;62:31–8. 8. Hadlock FP, Park SK, Awe RJ, et al. Unusual radiographic findings in adult pulmonary tuberculosis. AJR Am J Roentgenol. 1980;134:1015–8. 9. Berger HW, Samortin TG. Miliary tuberculosis: diagnostic methods with emphasis on the chest roentgenogram. Chest. 1970;58:586-9. 10. Jones BE, Ryu R, Yang Z, et al. Chest radiographic findings in patients with tuberculosis with recent or remote infection. Am J Respir Crit Care Med. 1997;156(4 Pt 1):1270–3. 11. Greenberg SD, Frager D, Suster B, et al. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance). Radiology. 1994;193:115–9. 12. Lee KS, Hwang JW, Chung MP, et al. Utility of CT in the evaluation of pulmonary tuberculosis in patients without AIDS. Chest. 1996;110:977–84. 13. Pereira BAF, Macêdo SGD, Nogueira RA, et al. Aspectos tomográficos da consolidação lobar na tuberculose pulmonar primária. Radiol Bras. 2009;42:109–13. 14. Hatipo?lu ON, Osma E, Manisali M, et al. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax. 1996;51:397–402. 15. Im JG, Itoh H, Shim YS, et al. Pulmonary tuberculosis: CT findings – early active disease and sequential change with antituberculous therapy. Radiology. 1993;186:653–60. 16. Leung AN. Pulmonary tuberculosis: the essentials. Radiology. 1999;210:307–22. 17. Bombarda S, Figueiredo CM, Seiscento M, et al. Estudo comparativo entre a radiografia e a tomografia computadorizada do tórax na forma ativa da tuberculose pulmonar. J Pneumol. 2000;26:S18. 18. Laissy JP, Cadi M, Boudiaf ZE, et al. Pulmonary tuberculosis: computed tomography and high-resolution computed tomography patterns in patients who are either HIV-negative or HIV-seropositive. J Thorac Imaging. 1998;13:58–64. 19. Haramati LB, Jenny-Avital ER, Alterman DD. Effect of HIV status on chest radiographic and CT findings in patients with tuberculosis. Clin Radiol. 1997;52:31–5. 20. Goodman PC. Tuberculosis and AIDS. Radiol Clin North Am. 1995;33:707–17. 21. Saurborn DP, Fishman JE, Boiselle PM. The imaging spectrum of pulmonary tuberculosis in AIDS. J Thorac Imaging. 2002;17:28–33. 22. Castañer E, Gallardo X, Mata JM, et al. Radiologic approach to the diagnosis of infectious pulmonary diseases in patients infected with the human immunodeficiency virus. Eur J Radiol. 2004;51:114–29. 23. Pereira-Silva JL, Kavakama J, Terra Filho M, et al. Consenso brasileiro sobre a terminologia dos descritores de tomografia computadorizada do tórax. J Bras Pneumol. 2005;32:149–56. 24. Jasmer RM, Gotway MB, Creasman JM, et al. Clinical and radiographic predictors of the etiology of computed tomography-diagnosed intrathoracic lymphadenopathy in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;31:291–8. 25. Hulnick DH, Naidich DP, McCauley DI. Pleural tuberculosis evaluated by computed tomography. Radiology. 1983;149:759–65. 26. Leung AN, Brauner MW, Gamsu G, et al. Pulmonary tuberculosis: comparison of CT findings in HIV-seropositive and HIV-seronegative patients. Radiology. 1996;198:687–91. 27. McGuinness G, Naidich DP, Jagirdar J, et al. High-resolution CT findings in miliary lung disease. J Comput Assist Tomogr. 1992;16:384–90. 28. Andreu J, Cáceres J, Pallisa E, et al. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol. 2004;51:139–49. 29. Aviram G, Fishman JE, Sagar M. Cavitary lung disease in AIDS: etiologies and correlation with immune status. AIDS Patient Care STDS. 2001;15:353–61. 30. McAdams HP, Erasmus J, Winter JA. Radiologic manifestations of pulmonary tuberculosis. Radiol Clin North Am. 1995;33:655–78. 31. Gutiérrez J, Miralles R, Coll J, et al. Radiographic findings in pulmonary tuberculosis: the influence of human immunodeficiency virus infection. Eur J Radiol. 1991;12:234–7. 1. Master, Director, Department of Diagnostic and Therapeutic Support of Instituto de Infectologia “Emílio Ribas” (IIER), São Paulo, SP, Brazil. 2. PhD, MD, Director, Unit of Imaging Diagnosis and Graphic Methods of Instituto de Infectologia “Emílio Ribas” (IIER), São Paulo, SP, Brazil. 3. PhD, MD, Radiologist responsible for the Unit of Imaging Diagnosis at Hospital Paulistano, São Paulo, SP, Brazil. 4. PhD, MD, Physician Assistant at Division of Pneumology, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil. 5. Private Docent, Technical Director, Instituto de Infectologia “Emílio Ribas” (IIER), São Paulo, SP, Brazil. 6. Private Docent, Head of Sector at the Service of Pathological Anatomy, Instituto de Infectologia “Emílio Ribas” (IIER), São Paulo, SP, Brazil. Mailing Address: Dra. Lanamar Aparecida de Almeida Avenida Doutor Arnaldo, 165, Pacaembu São Paulo, SP, Brazil, 01246-900 E-mail: lanamar.almeida@emilioribas.sp.gov.br Received August 26, 2010. Accepted after revision November 12, 2010. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554