Arterial spin labeling (ASL) is a recently developed magnetic resonance (MR) technique that assesses cerebral blood flow (CBF)(1,2). This method has been mainly used for investigative purposes with very few centers in the world performing it on a routine clinical basis. ASL has already been validated and proven to be useful in the assessment of a growing number of diseases and conditions(3). As with any recently established technique, ASL has some limitations that need to be overcome to become more widely used and to be part of the daily routine of the neuroimaging specialist.
Off line post processing is probably the single most important factor that has limited ASL accessibility. The low signal-to-noise ratio (SNR) is another inherent problem in ASL imaging. The lack of transit time information (using current methods), susceptibility artifacts derived from blood products, metal deposition, calcification and surgical material can potentially worsen this situation, as well as areas of interfaces between brain and air containing cavities (paranasal sinuses and mastoid air cells).
There are multiple ways of studying CBF including positron emission tomography, computed tomography (CT) perfusion, MR perfusion (susceptibility-weighted imaging or bolus tracking), single photon emission and xenon CT. The main advantage of ASL is the lack of the need for an injection of intravenous contrast material, which makes it a gadolinium-free method and injection device independent. Furthermore, ASL provides a measure of absolute blood flow (as opposed to relative cerebral blood volume from gadolinium based methods) (please refer to the Figure 1) and can also be repeated many times without the fear of nephrogenic systemic fibrosis, especially in patients with significant renal disease(4).
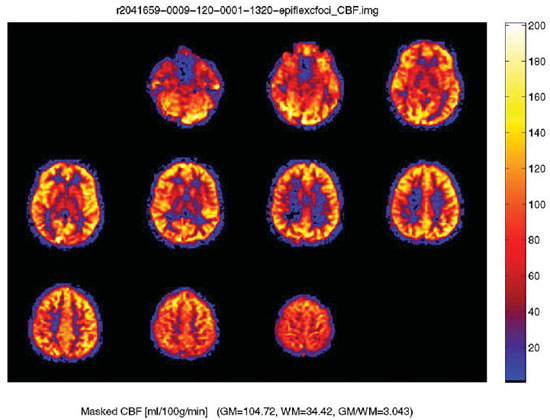
Figure 1. Normal arterial spin labeling JPEG map in a 30-year-old male with color ramp representing units of mL/100 g tissue/min. Arterial spin labeling or “gadolinium-free perfusion” is a technique in which there is quantitative measurement of the cerebral blood flow. The difference in cerebral blood flow between grey and white matter is readily seen.
Currently, four major current ASL techniques are available: pulsed ASL (PASL), continuous ASL (CASL), pseudo-continuous ASL (PCASL) and velocity-selective ASL (VSASL)(5).
In ASL imaging, the spins (protons) of the flowing blood supplying the brain are labeled (or tagged) before entering the region to be imaged. The extent of parenchyma to be studied and the location where the spins are labeled may vary with the different methods. Images are acquired in two phases: baseline (control) and post-labeling phases, with the time gap following the labeling tag known as “post-labeling delay”. The signal is obtained by subtracting the control from the post-labeling images. The resulting difference in signal is small and dependent on the cardiac output, travel time of the labeled spins to arrive in the area to be imaged and on the velocity of flow. To compensate for the low SNR, multiple sets of pre- and post-labeling images are typically acquired to ensure enough perfusion signal(5).
In PASL, the radiofrequency (RF) pulses used to label the spins are single and short (2- to 5 milliseconds) with a wider labeling area, located proximal to the imaging plane. PASL has the advantage of having higher tagging efficiency, less power deposition and improved transit time for the labeled spins to travel from the labeling area to the imaging plane. The main difficulties in PASL are low SNR and increased transit delay.
In CASL, the RF pulses are longer (1-2 seconds) and the labeling area narrower, located just below the imaging plane. In this technique, the spins are labeled continuously as they pass through the labeling area. This results in more SNR than in PASL, but more RF power deposition. Lower labeling efficiency and the necessity of continuous RF transmitting hardware are the main downsides of CASL.
PCASL was developed to have high labeling efficiency as with CASL, but with less RF power deposition. In comparison with CASL, this technique has a good trade-off between power deposition and SNR. PCASL has a higher SNR than PASL, higher labeling power than CASL, but presently has the disadvantage of being less clinically available.
VSASL has the ability to measure low flow. In comparison with PASL, CASL and PCASL, which label the spins at a specific location, this technique labels all the inflowing spins that are moving faster than a specific threshold. Disadvantages are lower SNR and difficulties in determining the specific encoding velocity(5).
Many pathological processes have been studied using ASL. Two major patterns are seen: hypo- and hyperperfusion, which can be focal or diffuse. Processes that demonstrate focally decreased perfusion include the ischemic core and penumbra, chronic cerebrovascular occlusive disease, chronic microvascular ischemia, encephalomalacia, hematoma, infection, arteriovenous malformation steal phenomena, posterior reversible encephalopathy syndrome (in the late phase) and post-ictal states. Diffusely decreased perfusion can be seen in brain death, poor cardiac output, aging with cerebral atrophy, vasculitis, cerebral vasospasm, as well as exogenous agents (caffeine)(6).
Focal hyperperfusion can be seen in conditions such as: luxury perfusion, posterior reversible encephalopathy syndrome (in the acute phase), reperfusion in ischemic areas, seizure activity, and in some cases of vascular malformations (including developmental venous anomalies). Diffuse increased perfusional states can be seen in normal individuals, global cerebral hyperperfusion complicating embolic stroke, carotid endarterectomy with the post-CEA hyperperfusion syndrome, traumatic head injury, and in the setting of hypercapnia. Brain tumors can demonstrate patterns of hypo- or hyperperfusion(7).
In conclusion, ASL is a promising and evolving technique used to measure CBF. This gadolinium-free MR perfusion method has several advantages including the lack of a contrast injection and the ability to provide absolute blood flow measurement. The absence of the risk of nephrogenic systemic fibrosis, non-invasiveness and the possibility of repeating the study several times if necessary, certainly will play a role in the popularization of this technique.
REFERENCES
1. Chalela JA, Alsop DC, Gonzalez-Atavales JB, et al. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–7.
2. Brown GG, Clark C, Liu TT. Measurement of cerebral perfusion with arterial spin labeling: Part 2. Applications. J Int Neuropsychol Soc. 2007;13:526–38.
3. Wintermark M, Sesay M, Barbier E, et al. Comparative overview of brain perfusion imaging techniques. J Neuroradiol. 2005;32:294–314.
4. Deibler AR, Pollock JM, Kraft RA, et al. Arterial spin-labeling in routine clinical practice, part 1: technique and artifacts. AJNR Am J Neuroradiol. 2008;29:1228–34.
5. Pollock JM, Tan H, Kraft RA, et al. Arterial spin-labeled MR perfusion imaging: clinical applications. Magn Reson Imaging Clin N Am. 2009;17:315–38.
6. Deibler AR, Pollock JM, Kraft RA, et al. Arterial spin-labeling in routine clinical practice, part 2: hypoperfusion patterns. AJNR Am J Neuroradiol. 2008;29:1235–41.
7. Deibler AR, Pollock JM, Kraft RA, et al. Arterial spin-labeling in routine clinical practice, part 3: hyperperfusion patterns. AJNR Am J Neuroradiol. 2008;29:1428–35.
1. MD, Visiting Fellow Advanced Neuroscience Imaging Research Core – Wake Forest University School of Medicine, Winston-Salem, NC, USA, Neuroradiology Clinical Fellow – McGill University Health Center, Montreal, Que., Canada.
2. Neuroradiologist, Professor and Chief of Neuroradiology – Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Corresponding Author:
Fabrício Guimarães Gonçalves
Wake Forest University Baptist Medical Center
Medical Center Boulevard
Winston-Salem, NC, USA 27157
E-mail: goncalves.neuroradio@gmail.com
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011
