Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 6 - Nov. / Dec. of 2010
Vol. 43 nº 6 - Nov. / Dec. of 2010
|
REVIEW ARTICLE
|
|
Usefulness of ultrasonography in children with suspected dengue hemorrhagic fever: a literature review* |
|
|
Autho(rs): Ricardo Villar Barbosa de Oliveira1; Lívia Teresa Moreira Rios2; Maria dos Remédios Freitas Carvalho Branco3; Leônidas Lopes Braga Júnior4; Janílson Moucherek Soares Nascimento5; Gilnara Fontinelle Silva6; Kemuel Pinto Bandeira7 |
|
|
Keywords: Dengue; Dengue hemorrhagic fever; Dengue shock syndrome; Pleural effusion; Ultrasonography. |
|
|
Abstract: INTRODUCTION
Dengue fever is an arbovirosis responsible for annual epidemics in Brazil. It is caused by one of the four serotypes of dengue virus (DEN1, DEN2, DEN3 and DEN4), with a clinical spectrum ranging from asymptomatic to severe, life-threatening conditions(1–5). Symptomatic dengue virus infection presents as a nonspecific febrile condition, as classical dengue, dengue hemorrhagic fever (DHF) with capillary extravasation that may progress to shock, or dengue shock syndrome that may be fatal if not appropriately treated(1). In Brazil, DHF incidence is highest in the adult population. A different pattern of distribution by age range has been observed over the last years in the Amazonas state, with highest rates of DHF in children < 15 years of age(2). Approximately 95% of cases occur in children < 15 years of age, and > 5% in infants(1,3). The most severe presentations of this disease are more prevalent in children than in adults(4). Increased capillary permeability is the main feature of DHF, represented by increased capillary permeability, with leakage of albumin out of the vascular space, leading to cavitary effusion and hemoconcentration with increase in the hematocrit levels described as polyserositis(5,6), classified into mild and severe, according to the World Health Organization criteria(1). Polyserositis is associated to hemorrhagic manifestations and thrombocytopenia (Figure 1). It is well established in the literature that, typically, the hypotension secondary to this plasma leakage occurs up to 48 hours after defervescence, the moment of fever abatement where the fever decreases to less than 38°C(7). 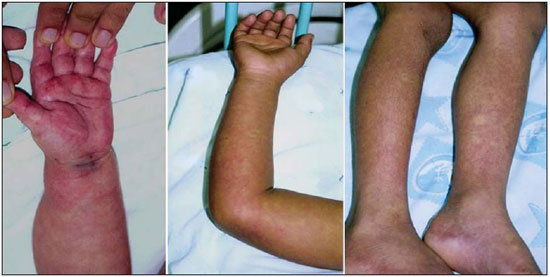 Figure 1. Hemorrhagic manifestations of mild and severe DHF. Despite the nonspecificity of sonographic findings, ultrasonography is useful for the early diagnosis in patients with DHF and for differential diagnosis of other febrile diseases(6). The objective of the present literature review is to describe the main sonographic findings and evaluating the role of ultrasonography in the assessment of children with suspected dengue hemorrhagic fever. MAIN SONOGRAPHIC FINDINGS Ultrasonography is a safe, low-cost imaging method that do not utilizes ionizing radiation, with high sensitivity to detect early signs of plasma leakage, many times in anticipation of the most critical period of the disease corresponding to the fever abatement to a temperature < 38°C, that is known as defervescence, where the risk for shock is higher. This critical phase extends from the third to the fifth day of febrile disease in children, where gallbladder wall thickening and cavitary effusion are observed in addition to severe abdominal pain, persistent emesis and increased hematocrit levels(5–15). The sonographic signs of plasma leakage, particularly pleural effusion, may be early identified, up to two days before defervescence, preceding changes in hematocrit levels(7). Sonographic findings express the increase in capillary permeability (a sign of plasma leakage) and include cavitary effusion (ascites, pleural and pericardial effusion), and gallbladder wall thickening present in one third of patients affected by the mild presentation, and in 95% of cases with the severe presentation of DHF. Additionally, the presence of fluid in the perirenal space can be visualized(6,8). Splenomegaly, hepatomegaly and volumetric increase of the pancreas may also be observed(6). Balasubramanian et al.(11), in a comparative analysis of extravasation parameters including clinical signs, hemoconcentration > 20%, hypoproteinemia, ultrasonography and chest radiography, concluded that ultrasonography was the best method for screening DHF, with 91.42% sensitivity and negative predictive value of 84.21%. Ascites, pleural effusion, gallbladder wall thickening and hepatomegaly were reported by the authors as predominant sonographic findings. During a dengue epidemic, the diagnosis of DHF should be considered as ultrasonography demonstrates gallbladder wall thickening, ascites, splenomegaly and pleural effusion in a febrile patient with thrombocytopenia(9,10). CAVITARY EFFUSION There is a correlation between pleural effusion, ascites, presence of fluid in the perirenal space, hepatic subcapsular collection and pericardial effusion with severity in cases of DHF in children(6,7). Pleural effusion is more commonly observed in DHF than in classical dengue, requiring rigorous observation(12). Pleural effusion is the most frequent sonographic finding in cases of plasma leakage, and is present, even subtlety, in children affected by classical dengue, where this abnormality is usually transitory and of rapid resolution(7). Most frequently, the onset of pleural effusion occurs immediately after defervescence, between the third and seventh day(12). In children, however, the onset of severe presentations is usually observed at about the third day, but not always is associated with defervescence(13). Some studies demonstrate that pleural effusion may be present up to one day before defervescence in some patients(7). It may be right unilateral or bilateral (Figures 2 and 3). It is rarely observed as left unilateral. Conventional chest radiography presents lower sensitivity than ultrasonography in the detection of minor pleural effusions(8,10,11,14). In another study, however, right lateral decubitus chest radiography performed one day after defervescence, has demonstrated higher sensitivity in the detection of pleural effusion as compared with ultrasonography. Its disadvantage is the higher exposure of the child to ionizing radiation(7). 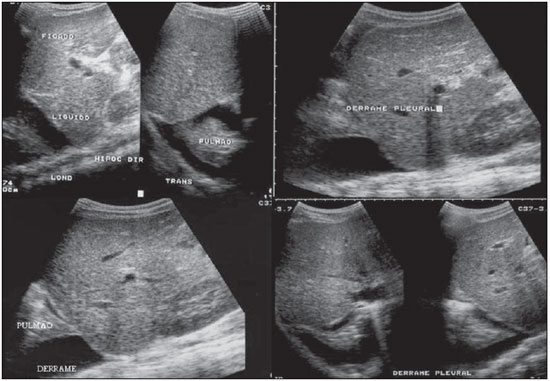 Figure 2. Pleural effusion at right, usual finding of plasma leakage, present even in a subtle presentation in children with classical dengue. In these cases, this finding is usually transitory and of rapid resolution. 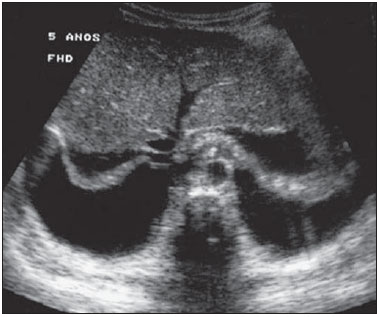 Figure 3. Plasma leakage expression as bilateral pleural effusion. Pericardial effusion is less frequently found, and may be observed in up to 28.5% of cases in assessments performed between the fifth and seventh febrile days(10,14). The presence of volumes as large as approximately 1,000 to 1,500 ml is necessary for clinical detection of free fluid, while ultrasonography can identify as little as about 100 ml(16). Ascites was detected in 26% to 34% of cases with mild DHF, and in 94% to 95% of cases with severe DHF(6,17) (Figure 4). 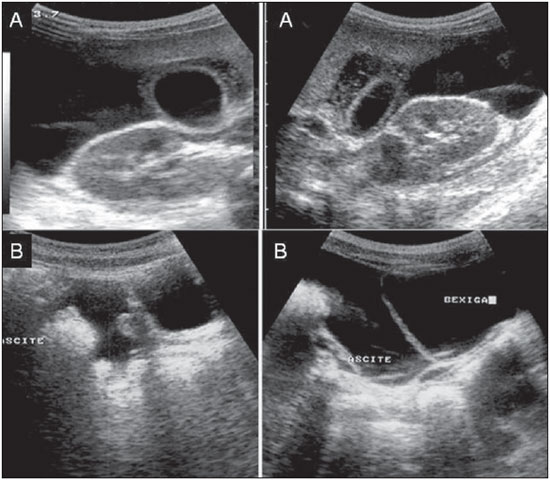 Figure 4. Presence of fluid in the abdominal cavity (ascites), in the anterior subhepatic space (A) and in the pelvic region (B). Hepatic subcapsular fluid is little evidenced, and its presence is a sign of disease severity. However, it is a transitory finding (only one to two days) that may be observed at around the fourth to the fifth day after the disease onset(6) (Figure 5). 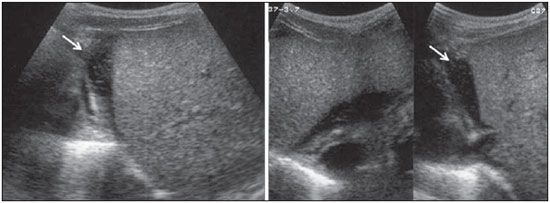 Figure 5. Presence of a small amount of hepatic subcapsular fluid demonstrated at about the fourth day of disease. The presence of fluid in the perirenal space could not be visualized in the cases of mild DHF. However, it could be seen in 77% of patients severely affected by DHF. Thus this is a significant marker for disease severity(6). GALLBLADDER WALL THICKENING Gallbladder wall thickening is a nonspecific finding frequently observed in other biliary or non-biliary conditions, such as acute cholecystitis, cirrhosis of liver, viral hepatitis, congestive heart failure, chronic kidney disease and hypoalbuminemia(6–9,17,18). Although the normal value for gallbladder wall thickness is still to be well established in the literature, such finding is considered in cases where the gallbladder wall thickness is > 3.0 mm. A more accurate measurement can be obtained on the anterior sub-hepatic wall in a longitudinal section, avoiding side lobe artifacts caused by the presence of adjacent intraluminal intestinal gas(18‑20). Four distinct gallbladder wall thickening patterns can be identified: a striated pattern of multiple hypoechoic layers separated by echogenic zones; asymmetric pattern with echogenic tissue projecting into the gallbladder lumen; a central hypoechogenic zone separated by two echogenic layers; and a uniform echogenic pattern(21). In patients with DHF, the striated pattern predominates (Figure 6), as a result of a probable fluid accumulation between the gallbladder wall layers producing striations, as a function of the osmotic intravascular pressure(10,21). 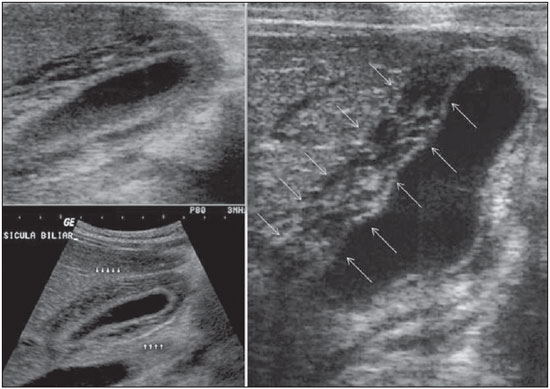 Figure 6. Longitudinal section of gallbladder, demonstrating a striated pattern of parietal thickening with multiple echogenic layers intermingled with fluid. Gallbladder wall thickening in children with DHF was first reported in 1991 by Pramuljo & Harun, in a study describing sonographic findings in 29 children with DHF, where 18% of cases presented gallbladder wall thickening(22). Gallbladder wall thickening was significantly associated with severe presentations of dengue, and with thrombocytopenia and hemoconcentration in suspicious cases of dengue. In some studies, this finding has been a relevant marker for clinical diagnosis and indicator of severity of DHF in children(17,21–24) (Figures 7 and 8). 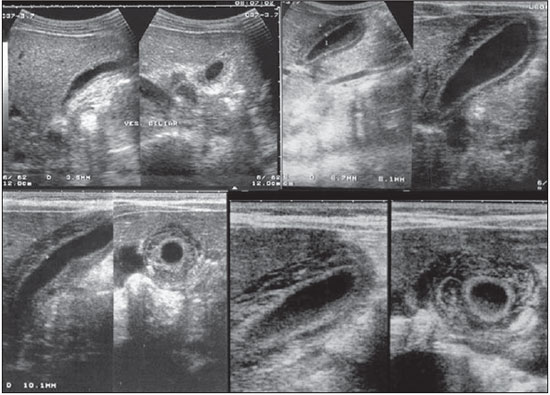 Figure 7. Different degrees of striated gallbladder wall thickening. 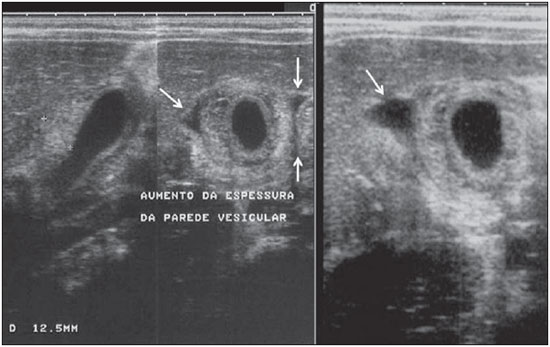 Figure 8. Significant thickening of gallbladder wall (arrow), presence of small amount of free, anechoic, homogeneous fluid surrounding the gallbladder. The earliest finding in the subgroup of children with classical dengue was gallbladder wall thickening. However, besides resolving faster, it is less frequently detected than pleural effusion. There is a higher probability of detection when the US scan is performed at the second or third febrile day(7,13). Two studies developed in Indonesia by Setiawan et al.(6,17) have found gallbladder wall thickening > 3.0 mm in one third (32% to 33%) of patients with the mild presentation of the disease and in most of those with severe disease (94% to 95%), allowing the authors to confirm to positive association between gallbladder wall thickening and the disease severity, and concluding that this finding can be utilized in the identification of patients with higher risk for progressing to shock. In the cases of DHF, gallbladder wall thickening > 3.0 mm and < 5.0 mm presents a sensitivity of 93.8% and may be utilized as a criterion for patients’ hospitalization and monitoring. In cases where the gallbladder wall thickness is > 5.0 mm, the specificity achieves 91.7%, a threshold that can be utilized in the selection of patients with higher risk for progressing to shock(17). Venkata Sai et al.(10) have evaluated 88 children in the age range between two and nine years, with serological tests positive for dengue, and have demonstrated gallbladder wall thickening in 100% of the patients, evidenced by ultrasonography performed in the period between the second and seventh febrile day. This finding was followed by pleural effusion, observed with higher frequency from the fifth febrile day. Then, the authors concluded that, during an epidemic outbreak, gallbladder wall thickening, either with or without signs of polyserositis in a febrile patient, should suggest the possibility of classical dengue/DHF. The value of gallbladder wall thickening as an ancillary factor in the diagnosis and prognosis of children with DHF has also been corroborated by other authors(23–25). Although this finding is nonspecific and may be present in other febrile diseases, it is useful in the early diagnosis and prediction of severity in cases of DHF, identifying the patients with higher risk for progressing to shock. The sonographic measurement of the gallbladder wall thickness is significantly associated with the severe presentations of dengue, and can also be utilized as a marker for thrombocytopenia and hemoconcentration. Thus, ultrasonography is a relevant method as a prognostic test in the severe presentations of DHF in children. Chacko & Subramanian, in a study evaluating 59 children with diagnosis of dengue shock syndrome, have reported that the presence of ascites and pleural effusion were the most predictive indicators of shock; on the other hand, gallbladder wall thickening was not associated with the presence of shock, as reported in previous studies(26). VOLUMETRIC INCREASE OF ORGANS Volumetric increase of organs is a nonspecific finding, corresponding to enteric findings of dengue, and should be taken into consideration in both clinical and sonographic context of plasma leakage(10). Hepatomegaly, splenomegaly and, less frequently, volumetric increase of the pancreas have been described in several studies, but these findings are observed with a similar frequency in the mild and severe DHF presentations, with highest incidence of hepatomegaly(6,10,11). CONCLUSION In children with suspected dengue hemorrhagic fever, ultrasonography, although nonspecific, is a relevant ancillary tool for the early diagnosis of plasma leakage signs and for prediction of the disease severity, identifying mild and severe cases of DHF, besides contributing in the differential diagnosis with other causes of febrile disease. REFERENCES 1. Organização Mundial da Saúde. Dengue hemorrágica: diagnóstico, tratamento, prevenção e controle. São Paulo, SP: Livraria Santos Editora; 2001. 2. Siqueira Júnior JB, Martelli CMT, Coelho GE, et al. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerg Infect Dis. 2005;11:48–53. 3. Torres EM. Dengue. Rio de Janeiro, RJ: Editora Fiocruz; 2005. 4. Guzmán MG, Kourí G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. 5. Vabo KA, Torres Neto G, Santos AASMD, et al. Achados ultra-sonográficos abdominais em pacientes com dengue. Radiol Bras. 2004;37:159–62. 6. Setiawan MW, Samsi TK, Wulur H, et al. Dengue haemorrhagic fever: ultrasound as an aid to predict the severity of the disease. Pediatr Radiol. 1998;28:1–4. 7. Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, et al. Natural history of plasma leakage in dengue hemorrhagic fever: a serial ultrasonographic study. Pediatr Infect Dis J. 2007;26:283–90. 8. Thulkar S, Sharma S, Srivastava DN, et al. Sonographic findings in grade III dengue hemorrhagic fever in adults. J Clin Ultrasound. 2000;28:34–7. 9. Wu KL, Changchien CS, Kuo CH, et al. Early abdominal sonographic findings in patients with dengue fever. J Clin Ultrasound. 2004;32:386–8. 10. Venkata Sai PM, Dev B, Krishnan R. Role of ultrasound in dengue fever. Br J Radiol. 2005;78:416–8. 11. Balasubramanian S, Janakiraman L, Kumar SS, et al. A reappraisal of the criteria to diagnose plasma leakage in dengue hemorrhagic fever. Indian Pediatr. 2006;43:334–9. 12. Firmida MC. Derrame pleural na criança com dengue. Acta Scientiae Medica. 2008;1:35–43. 13. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Dengue: diagnóstico e manejo clínico – adulto e criança. 3ª ed. Brasília, DF: Ministério da Saúde; 2007. 14. Pelupessy JM, Allo ER, Jota S. Pericardial effusion in dengue haemorrhagic fever. Paediatr Indones. 1989;29:72–5. 15. Quiroz-Moreno R, Méndez GF, Ovando-Rivera KM. Utilidad clínica del ultrasonido en la identificación de dengue hemorrágico. Rev Med Inst Mex Seguro Soc. 2006;44:243–8. 16. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by ultrasound. Radiology. 1970;96:15–22. 17. Setiawan MW, Samsi TK, Pool TN, et al. Gallbladder wall thickening in dengue hemorrhagic fever: an ultrasonographic study. J Clin Ultrasound. 1995;23:357–62. 18. Patriquin HB, DiPietro M, Barber FE, et al. Sonography of thickened gallbladder wall: causes in children. AJR Am J Roentengol. 1983;141:57–60. 19. Handler SJ. Ultrasound of gallbladder wall thickening and its relation to cholecystitis. AJR Am J Roentgenol. 1979;132:581–5. 20. Laing FC, Federle MP, Jeffrey RB, et al. Ultrasonic evaluation of patients with acute right upper quadrant. Radiology. 1981;140:449–55. 21. Teefey SA, Baron RL, Bigler SA. Sonography of the gallbladder: significance of striated (layered) thickening of the gallbladder wall. AJR Am J Roentgenol. 1991;156:945–7. 22. Pramuljo HS, Harun SR. Ultrasound findings in dengue haemorrhagic fever. Pediatr Radiol. 1991;21:100–2. 23. Gupta S, Singh SK, Taneja V, et al. Gall bladder wall edema in serology proven pediatric dengue hemorrhagic fever: a useful diagnostic finding which may help in prognostication. J Trop Pediatr. 2000;46:179–81. 24. Sehgal A, Gupta S, Tyagi V, et al. Gall bladder wall edema is not pathogenic of dengue infection. J Trop Pediatr. 2002;48:315–6. 25. Colbert JA, Gordon A, Roxelin R, et al. Ultrasound measurement of gallbladder wall thickening as a diagnostic test and prognostic indicator for severe dengue in pediatric patients. Pediatr Infect Dis J. 2007;26:850–2. 26. Chacko B, Subramanian G. Clinical, laboratory and radiological parameters in children with dengue fever and predictive factors for dengue shock syndrome. J Trop Pediatr. 2008;54:137–40. 1. Master, Coordinator for the Service of Pediatric Imaging, Hospital Universitário da Universidade Federal do Maranhão (HUUFMA), São Luís, MA, Brazil. 2. Master, Coordinator for the Imaging Center of Service of Obstetrics and Gynecology, Hospital Universitário da Universidade Federal do Maranhão (HU-UFMA), São Luís, MA, Brazil. 3. Fellow PhD degree, Associate Professor, Division of Infectious and Parasitic Diseases, Course of Medicine, Universidade Federal do Maranhão (UFMA), São Luís, MA, Brazil. 4. MD, Specialist in Pediatrics, Physician at Unit of Pediatrics, Hospital Universitário da Universidade Federal do Maranhão (HUUFMA), São Luís, MA, Brazil. 5. MD, Specialist in Ultrasonography, Physician at Service of Pediatric Imaging, Hospital Universitário da Universidade Federal do Maranhão (HU-UFMA), São Luís, MA, Brazil. 6. Graduate Student of Medicine, Faculdade de Medicina da Universidade Federal do Maranhão (UFMA), São Luís, MA, Brazil. 7. Titular Member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), MD, Service of Pediatric Imaging, Hospital Universitário da Universidade Federal do Maranhão (HUUFMA), São Luís, MA, Brazil. Mailing Address: Dra. Lívia Teresa Moreira Rios Avenida do Vale, L-10, Q-35 Ed. Costa Rica, ap. 801, Jardim Renascença São Luís, MA, Brazil, 65075-820 E-mail: ltlrios@terra.com.br Received February 12, 2010. Accepted after revision August 18, 2010. * Study developed in the Unit of Pediatrics at Hospital Universitário da Universidade Federal do Maranhão (HU-UFMA), São Luís, MA, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554