Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 6 - Nov. / Dec. of 2010
Vol. 43 nº 6 - Nov. / Dec. of 2010
|
ORIGINAL ARTICLE
|
|
Reliability of qualitative assessment of brain magnetic resonance imaging in extremely premature infants* |
|
|
Autho(rs): Andre Dietz Furtado1; Marcus Vinicius Rocha Pinto2; Cláudio de Carvalho Rangel3; Luiz Celso Hygino da Cruz Jr4; José Maria A. Lopes5; Manoel de Carvalho5; Jofre Antônio Oliveira Cabral5; Romeu Côrtes Domingues6; Emerson Leandro Gasparetto7 |
|
|
Keywords: Preterm; Brain; Magnetic resonance imaging; Imaging; Hypoxia. |
|
|
Abstract: INTRODUCTION
Over the last decades, there have been significant advances in perinatal and neonatal care, which have dramatically improved survival rates for infants with very low birth weights. Nevertheless, there has been a increasing concern regarding the later neurodevelopmental challenges faced by surviving infants(1). The developing brain is highly vulnerable to injury from a variety of ischemic, inflammatory, infective, and neurotoxic factors(2). Extremely preterm infants are at higher risk of brain hemorrhage, white matter (WM) lesions, and poor cerebral development(3). The central nervous system (CNS) damage increases the probability of neurological and developmental disabilities in this group of patients(4). Conventional neuroimaging is usually employed to assess the presence and the extent of brain injury as well as to predict neurodevelopmental outcomes. The two major neuroimaging modalities used in the evaluation of the premature infant’s brain are cranial ultrasonography (US) and magnetic resonance imaging (MRI)(5–8). Cranial US is reliable in the evaluation of hemorrhagic lesions, hydrocephalus, and cystic changes(5,6). Such technique, however, is typically obtained through the anterior fontanel, which has a limited field of view. Moreover, cranial US is not accurate enough to evaluate diffuse or subtle brain injuries, specially in the WM(5–8). MRI of the brain is more sensitive and specific than cranial US to detect hemorrhage, ischemia, and WM lesions(5,6,9–11). It also provides better characterization of the most common brain abnormalities in preterm infants(5,6,12). The aim of the present study was to evaluate the reliability of the qualitative visual assessment of brain abnormalities using conventional brain MRI in a cohort of 45 extremely preterm infants. MATERIALS AND METHODS Patient population The Institutional Review Board of our Hospital approved the study and term of free and informed consent to perform MRI under sedation at term-equivalent age was obtained from the parents. A cohort of 45 consecutive infants with gestational age of 30 weeks or less (median of 27 weeks, ranging from 25 to 30 weeks) was enrolled in this prospective study. The gestational age was calculated from the date of the last menstrual period and confirmed with data from early US scans. The patients had a median birth weight of 890 g (ranging from 385 g to 1225 g). Critically ill patients were not considered for the study, at least until the neonatologist in charge of these patients confirmed that they could be submitted to MRI study at our imaging center affiliated to the hospital with no risk for the patient. Imaging protocol All the patients underwent MRI in a 1.5 T scanner (Magnetom Avanto – Siemens Medical Systems; Erlangen, Germany) using a head coil. The following sequences were obtained: T1-weighted sagittal three-dimensional gradient-echo (repetition time (TR)/echo time (TE) = 1770/3.9 ms, field of view (FOV) = 190 Χ 190 mm, matrix = 256 Χ 256, slice thickness = 0.7 mm), T2-weighted axial fast spin-echo (FSE) (TR/TE = 5610/159 ms, FOV = 180 Χ 180 mm, matrix = 256 Χ 256, slice thickness = 4 mm), and T2-weighted axial gradient-echo (TR/TE = 786/35 ms, FOV = 180 Χ 180 mm, matrix = 256 Χ 256, slice thickness = 4 mm, flip angle = 30°). Imaging analysis All the MRI studies were independently and blindly reviewed by two neuroradiologists (five and six years of experience). In cases of disagreement, a third neuroradiologist reviewed the images and final decisions were defined by consensus. The following MRI findings were evaluated: 1) diffuse and excessive high-signal intensity (DEHSI), defined as areas of abnormal, diffuse high-signal on T2-weighted FSE images within the periventricular and/or subcortical WM (signal intensity similar to the CSF)(7); 2) dilated lateral ventricles, if the transverse ventricular diameter was >10 mm, measured at the level of the atria(13–15), presence of intracranial hemorrhage defined as abnormal areas with signal characteristics compatible with blood products, classified according to its location in 3) intraparenchymal hemorrhage (IPH), 4) intraventricular hemorrhage (IVH), and 5) germinal matrix hemorrhage (GMH); 6) areas of abnormal signal in the basal ganglia and cortex; 7) cystic-like areas; 8) ventricular deformities; 9) enlargement of subarachnoid spaces overlying the cortical convexities (> 3 mm); 10) enlargement of the interhemispheric fissure (> 3 mm); 11) early foci of leukoencephalomalacia; 12) and gyri abnormalities(7). Statistical analysis Interobserver agreement in the MR images analysis was assessed with calculation of the kappa (κ) index, and the following ranges for agreement were used: 0.00, poor; 0.00 to 0.20, slight; 0.21 to 0.40, fair; 0.41 to 0.6, moderate; 0.61 to 0.8, substantial; and 0.81 to 1.0, almost perfect(16). The p value of less than 0.05 was considered as statistically significant. RESULTS Prevalence of brain MRI abnormalities in extreme preterm infants Among the 45 studied cases, only four patients (8.9%) had normal MR images (Table 1). The remaining 41 patients (91.1%) presented abnormalities on the MRI. The most common findings were DEHSI in the WM (75.6%) (Figure 1), lateral ventricles dilatation (42.2%) (Figure 2), GMH (31.1%) (Figure 3), IVH (28.9%) (Figure 4), ventricular deformities (24.4%) (Figure 5), and enlargement of the subarachnoid spaces (22.2%).  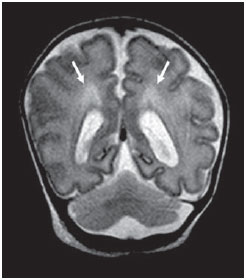 Figure 1. Coronal T2WI (5610/159) image demonstrates diffuse and excessive high-signal intensity, evidenced as areas of abnormal high-signal intensity within the periventricular white matter. 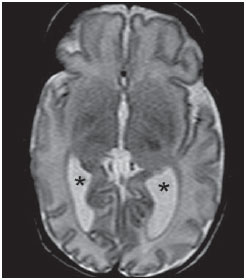 Figure 2. Axial T2WI (5610/159) image demonstrates lateral ventricles dilation measured at the level of ventricular atria (> 10 mm) (*). 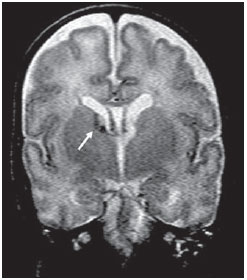 Figure 3. Coronal T2WI (5610/159) image demonstrates germinal matrix hemorrhage evidenced as low-signal intensity material in the subependymal zone (arrow). 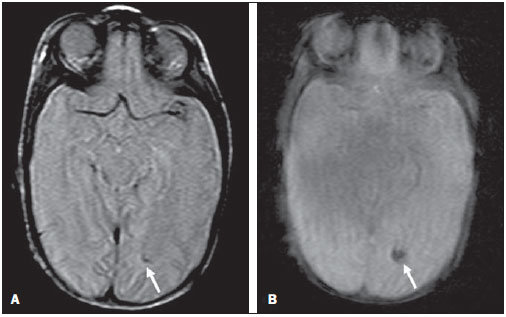 Figure 4. (A) Axial T2WI (5610/159) and (B) axial gradient-echo image (1770/3.9; flip angle = 30°) demonstrate low-signal intensity material layering in the occipital horn of the left lateral ventricle (arrows). Note marked blooming of the susceptibility effect from blood products on the gradient-echo image. 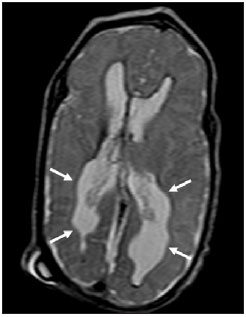 Figure 5. Axial T2WI (5610/159) image demonstrates ventricular deformities evidenced as irregular ventricular walls (arrows). Reliability of qualitative visual assessment of conventional brain MRI All the κ values obtained in the analysis of the interobserver variability were statistically significant. The interobserver agreement was high (κ > 0.60) for most of the abnormal MRI findings (Table 1). The κ statistic value was moderate for enlargement of the subarachnoid spaces overlying the cortical convexities (κ = 0.52) and was only fair for DEHSI in the WM (κ = 0.39). DISCUSSION Extremely preterm infants are at a high risk for adverse neurodevelopmental outcomes(17–19). Previous studies have evaluated the main brain abnormalities seen at MRI performed in these patients at term-equivalent age(6,7,12,20–23). The prevalence of brain MRI abnormalities was high in our patients cohort. Only four patients (8.9%) had normal brain MRI scans. The most common abnormalities were DEHSI in the WM (75.6%), ventricular dilation (42.2%), and hemorrhagic injury (GMH: 31.1% and IVH: 28.9%). This high prevalence of brain abnormalities observed in the present study with 45 extremely preterm infants is similar to previous studies(7,22). Cranial US is less sensitive to demonstrate most of these WM abnormalities. Moreover, the significance of the US finding of WM echogenicity is controversial(1,5–9,20,21). Inder et al.(21) have compared serial cranial US and brain MRI at term in a cohort of 96 extremely preterm infants. These authors emphasized the significant limitations of US for the detection of non-cystic WM injuries. Additionally, cranial US failed to demonstrate subtle WM injuries between birth and term in a group of 32 preterm infants as compared with brain MRI(5). In conclusion, US seems to have poorer sensitivity and specificity for the evaluation of WM abnormalities in extremely preterm infants as compared with MR imaging, and does not correlate with the clinical outcome(5,21). Brain MRI is, thus, considered as the main imaging modality to predict neurodevelopment outcome in extremely preterm infants. It has been suggested that the severity of conventional MRI abnormalities is directly related to adverse long-term neurodevelopmental outcomes(20). In our study, we assessed the reliability of the subjective visual assessment of conventional brain MRI performed at term-equivalent age in a cohort of 45 extremely preterm infants. The interobserver agreement was high (κ > 0.60) for most of the MRI findings, with the exception of the DEHSI in the WM. In our study, we observed a relatively low κ statistic value for DEHSI in the WM (κ = 0.39). Even though previous papers have defined the MR signal characteristics of DEHSI in the WM, it is still highly dependent on the radiologist’s experience(1,7,22,23). A thorough knowledge of the normal patterns of myelinization is essential when considering the possibility of DEHSI in the WM. Additionally, in some cases, CNS lesions were very severe and occurred early in the fetal development, making the evaluation of normal myelinization patterns even more difficult. This finding of relatively low interobserver agreement for WM DEHSI in our study may have relevant clinical implications because WM abnormalities have been reported as the main brain MRI abnormality related to the long- and short-term prognosis of extremely preterm infants; and clinical therapeutic decisions are frequently based on whether WM abnormalities are present or not(5,20). We acknowledge some limitations to our study. Critically ill patients were not considered for the study to avoid additional risk for them. MRI was performed at the term‑equivalent age, but not at birth, which is similar to other studies(6,7,12,20–23). Finally, as the aim of our study was to assess the reliability of brain MRI abnormalities, we did not correlate the MRI findings with the long-term outcome, which has already been studied(20). In conclusion, conventional MRI is usually employed to assess brain abnormalities in extremely preterm infants. The most common brain MRI findings at term-equivalent age in extremely preterm infants were DEHSI in the WM, ventricular dilation, GMH, and IVH, which have been associated with adverse neurodevelopmental outcome. According to the present study, conventional MRI seems to be a reliable method for evaluating the most common brain abnormalities in extremely premature infants; however, it seems that the presence of DEHSI in the WM is a less reliable finding. REFERENCES 1. Inder TE, Wells SJ, Mogridge NB, et al. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–9. 2. Kuban KC, Leviton A. Cerebral palsy. N Engl J Med. 1994;330:188–95. 3. Vohr BR, Allen M. Extreme prematurity – the continuing dilemma. N Engl J Med. 2005;352:71–2. 4. Wood NS, Marlow N, Costeloe K, et al. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343:378–84. 5. Maalouf EF, Duggan PJ, Counsell SJ, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107:719–27. 6. Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94. 7. Maalouf EF, Duggan PJ, Rutherford MA, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr. 1999;135:351–7. 8. Hope PL, Gould SJ, Howard S, et al. Precision of ultrasound diagnosis of pathologically verified lesions in the brains of very preterm infants. Dev Med Child Neurol. 1988;30:457–71. 9. Blankenberg FG, Norbash AM, Lane B, et al. Neonatal intracranial ischemia and hemorrhage: diagnosis with US, CT, and MR imaging. Radiology. 1996;199:253–9. 10. Blankenberg FG, Loh NN, Bracci P, et al. Sonography, CT, and MR imaging: a prospective comparison of neonates with suspected intracranial ischemia and hemorrhage. AJNR Am J Neuroradiol. 2000;21:213–8. 11. Barkovich AJ, Westmark K, Partridge C, et al. Perinatal asphyxia: MR findings in the first 10 days. AJNR Am J Neuroradiol. 1995;16:427–38. 12. Battin MR, Maalouf EF, Counsell SJ, et al. Magnetic resonance imaging of the brain in very preterm infants: visualization of the germinal matrix, early myelination, and cortical folding. Pediatrics. 1998;101:957–62. 13. Farrell TA, Hertzberg BS, Kliewer MA, et al. Fetal lateral ventricles: reassessment of normal values for atrial diameter at US. Radiology. 1994;193:409–11. 14. McArdle CB, Richardson CJ, Nicholas DA, et al. Developmental features of the neonatal brain: MR imaging. Part I. Gray-white matter differentiation and myelination. Radiology. 1987;162(1 Pt 1):223–9. 15. McArdle CB, Richardson CJ, Nicholas DA, et al. Developmental features of the neonatal brain: MR imaging. Part II. Ventricular size and extracerebral space. Radiology. 1987;162(1 Pt 1):230–4. 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. 17. Saigal S, Feeny D, Rosenbaum P, et al. Self-perceived health status and health-related quality of life of extremely low-birth-weight infants at adolescence. JAMA. 1996;276:453–9. 18. Hack M, Flannery DJ, Schluchter M, et al. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–57. 19. Marlow N, Wolke D, Bracewell MA, et al. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. 20. Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. 21. Inder TE, Anderson NJ, Spencer C, et al. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24:805–9. 22. Arthur R. Magnetic resonance imaging in preterm infants. Pediatr Radiol. 2006;36:593–607. 23. Valkama AM, Pääkkö EL, Vainionpää LK, et al. Magnetic resonance imaging at term and neuromotor outcome in preterm infants. Acta Paediatr. 2000;89:348–55. 1. Clinical Fellow in Neuroradiology, Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA. 2. Graduate Student of Medicine, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. 3. Radiologist, CDPI – Clínica de Diagnóstico Por Imagem and Clínica Multi-Imagem, Rio de Janeiro, RJ, Brazil. 4. Radiologist, CDPI – Clínica de Diagnóstico Por Imagem and Clínica Multi-Imagem, Fellow PhD degree, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. 5. Neonatologist, Clínica Perinatal Laranjeiras, Rio de Janeiro, RJ, Brazil. 6. Radiologist and Medical Director, CDPI – Clínica de Diagnóstico Por Imagem and Clínica Multi-Imagem, Rio de Janeiro, RJ, Brazil. 7. Radiologist, CDPI – Clínica de Diagnóstico Por Imagem and Clínica Multi-Imagem, Associated Professor at Department of Radiology of Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. Mailing Address: Dr. Emerson L. Gasparetto Avenida das Américas, 4666, sala 325, Barra da Tijuca Rio de Janeiro, RJ, Brazil, 22640-102 Email: egasparetto@gmail.com Received August 26, 2010. Accepted after revision October 21, 2010. * Study developed at CDPI – Clínica de Diagnóstico Por Imagem, Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554