Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 5 - Sep. / Oct. of 2010
Vol. 43 nº 5 - Sep. / Oct. of 2010
|
CASE REPORT
|
|
Paget’s disease with sacral involvement: a case report |
|
|
Autho(rs): Fernanda Nogueira Holanda Ferreira Braga1; Marcio Vale Braga2; Francisco Andrade Neto3 |
|
|
Keywords: Osteitis deformans; Paget’s disease of bone; Sacrum; Metabolic bone diseases; Bone resorption. |
|
|
Abstract: INTRODUCTION
Paget’s disease is a common focal, progressive osteometabolic disorder of bone remodeling characterized by both an increase in osteoclastic resorption and secondary bone formation, resulting in a disorganized and fragile lamellar bone mosaic susceptible to deformities and fractures(1–3). The authors report the case of a patient with sacral involvement by Paget’s disease and describe the respective radiological findings. CASE REPORT A male 71-year-old patient complaining of acute moderate pain in the left coxofemoral region, with ill-defined irradiation to the left thigh, in association with mechanical lumbar pain. The patient denied other symptoms, and the clinical examination did not demonstrate any alterations. The physical examination of the joint revealed a positive Patrick’s test on the left coxofemoral joint, with pain at palpation at a point near the trochanteric bursa. Lasègue’s maneuver was negative. Absence of pain at flexion, extension and palpation of the lumbar spine. Normal reflexes. Preserved muscular strength and sensitivity. Serum tests demonstrated the following results: alkaline phosphatase (ALP), 366.5 U/L (reference value: 129 U/L); ALP bone fraction, 287.8 U/L (80%); 24-hour calciuria, 410 mg (reference value: up to 220 mg). The hip radiograph is shown on Figure 1, bone scintigraphy on Figure 2, multislice computed tomography (CT) of the sacrum on Figure 3, and Figure 4 shows pelvic magnetic resonance imaging (MRI), with their respective findings. 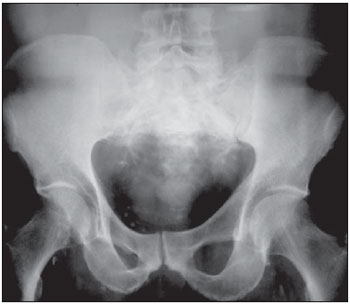 Figure 1. Plain hip radiography, anteroposterior view demonstrating areas of bone demineralization in the sacrum, with marginal sclerosis in the sacroiliac joints. 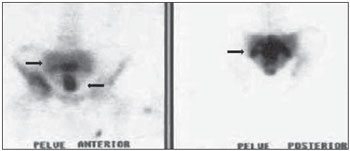 Figure 2. Whole-body Tc-99m scintigraphy demonstrates heterogeneous radiopharmaceutical distribution in the skeleton, with increased tracer uptake in the sacrum (arrows), right acetabulum (anterior pelvis) and bilaterally in the sacroiliac region (posterior pelvis). 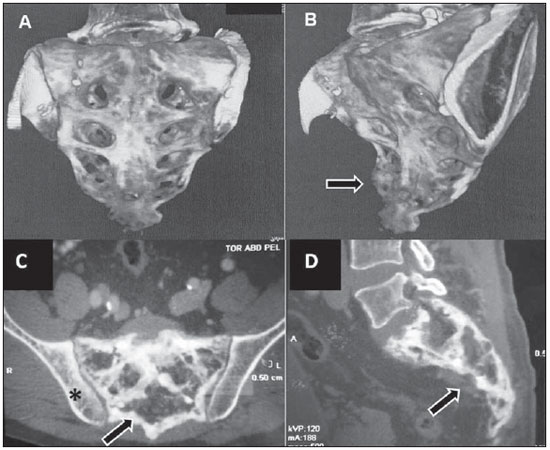 Figure 3. A,B: 3D multislice CT image. C,D: Respectively, axial and sagittal sections of the sacrum. Tomographic sections demonstrating significant enhancement of gross, medullary bone trabeculate in the sacrum, with a predominantly sclerotic pattern (arrows), loss of the normal trabecular architecture, and with no evidence of significant destruction of bone cortical or adjacent extraosseous soft tissues. On C, image demonstrating the right iliac involvement (asterisk) adjacent to the sacrum, with the previously mentioned characteristics. No evidence of fracture trace is observed. 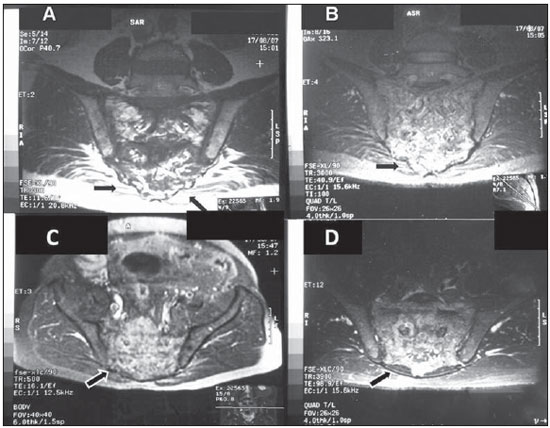 Figure 4. A,B: Coronal MRI sections demonstrating extensive sacral bone marrow replacement, including posterior elements with heterogeneous signal intensity on T1- (A) and T2- weighted (B) sequences in association with appearance of bone insufflations, and with narrowing of the rachidian canal. Such lesion narrows the sacral bone cortical (arrows) with discontinuity in at least one point in the right joint margin, with intra-articular extension. On C, the image shows an axial section demonstrating hyperintense signal on T1- weighted sequences with fat suppression (arrow). On D, the image demonstrates an axial T2-weighted sequence with hyperintense signal and gadolinium enhancement of perisacral soft tissues. Sacral biopsy was requested, considering the clinical-radiological suspicion of bone Paget’s disease in an elderly, male patient with ill-defined pain, and with the objective of ruling out the presence of associated neoplasia(3–5) as well as histopathologically confirming the presence of the disease. The histopathological analysis demonstrated irregular, thickened bone trabeculae forming a meshwork of intercommunicating spaces with remodeling changes, showing ossification lines with different orientations, mosaic pattern, with medullary, peritrabecular fibrosis and osteoclastic cells suggestive of Paget’s disease. Absence of malignancy is observed. The treatment selected was oral ibandronate 150 mg monthly doses. After six months of treatment, the patient presented reduction in total ALP levels to 132 U/L (a 63.98% reduction), and in the ALP bone fraction to 51 U/L (an 82.27% reduction), resulting in clinical management of the disease(1). After eight-month treatment, CT follow- up demonstrated the management of the disease progression. Bone scintigraphy was similar to the pre-treatment scintigraphic study. MRI demonstrated a small decrease in contrast uptake in the sacral region. Contrast uptake was not observed in the posterior acetabular column as well as in the adjacent soft tissues. DISCUSSION Paget’s disease is a chronic osteometabolic disorder characterized by abnormalities in all the phases of bone remodeling, resulting from osteoclastic and osteoblastic hyperactivity(1–4). The axial skeleton is most frequently affected, the pelvis being involved in about 70–90% of cases, and the spine in up to 53% of cases(3,4). Vertebral spine involvement occurs at one or more levels, with the lumbar level being described in up 58% of cases, thoracic level, in 45%, and cervical level, in 44%(3,4). However, in many cases the disease is asymptomatic and is incidentally diagnosed by radiological findings in studies performed for other reasons(3). Paget’s disease of bone is currently described as the second most prevalent osteometabolic disease worldwide, after osteoporosis(1–4). The most frequent symptom is bone or joint pain, with a prevalence in about 50% of cases(1,3), primarily explained by secondary degenerative joint disease, periosteal or cartilaginous disease, microfractures and bone deformities. Other less described causes for pain are pathological fracture, nervous compression, intervertebral involvement, spondylolysis, spondylolisthesis and sarcomatous degeneration(1–4). Periosteal apposition with endosteal absorption constitute the most frequent mechanism leading the affected bone to expand, which can be demonstrated by radiological imaging. In this setting, osteoclasts are increased in number and size. There is an osteoblastic dysfunction, with hyperactive, although morphologically normal, osteoblasts, leading to a chaotic development of a new periosteal and endosteal bone, with loss of the normal lamellar pattern(1–3). At the initial lytic phase, radiography of the involved bones demonstrates well-defined radiolucent areas without sclerosis. With the disease progression, at the mixed phase, gross trabeculae and cortical thickening are observed. At the final, blastic phase, areas of sclerosis and increased bone volume are observed(3–5). In this phase, the main differential diagnosis is with metastasis, particularly in breast and prostate(5). CT is better to demonstrate the loss of normal bone trabeculae, also with focal areas of lysis, sclerosis and cortical bone expansion as reported in the present case(3,4). In the present case, plain hip radiography did not show to be sufficiently elucidative for the diagnosis, leading the assisting physician to supplement the investigation with the other imaging resources reported and described in the literature(1–8). Tc-99m scintigraphy is highly sensitive, but poorly specific for detecting bone remodeling, demonstrating focal intensity with radiopharmaceutical uptake in the affected bones, suggesting the presence of disease even before the symptoms onset(1,4–6). MRI is indicated to investigate differential diagnoses such as malignancy, and to evaluate medullary infiltration in the vertebral presentation of the disease(3,5,7,8). Hyperintense signal corresponding to an increase in fat within the medullary space of the involved bone may be identified at all MRI sequences, characterizing an atrophic bone marrow, with a possible narrowing of the canal caused by the cortical thickening. Heterogeneous signal intensity is another pattern that can be found on T1- and T2-weighted sequences. On the T1- weighted sequence, the bone marrow may present decreased signal intensity, with foci of normality, which rules out the presence of malignant degeneration because of the absence of expansile lesions. On the other hand, T2–weighted sequence demonstrates heterogeneously hyperintense signal in the bone marrow, corresponding to its fibrovascular component. Finally a third presentation is identified by the low signal intensity of the bone marrow at all the sequences, representing sclerosis and characterizing the inactive blastic phase. The medullary enhancement can be seen with the utilization of gadolinium-based contrast agent(4,5,7,8). Bone biopsy is useful to confirm the diagnosis in cases of dubious lesion presentation, as well as in patients with high risk for developing associated neoplasias, either as a primary bone tumor or as a metastasis in the vertebral spine(3,4). Neoplastic transformation of the lesions, although rare, are considered as a complication of Paget’s disease, so bone biopsy becomes essential in this differentiation(3,4,8). Currently, bisphosphonates constitute the medication of choice for the specific treatment in an attempt to re-establish the normal bone remodeling, reducing the bone turnover(1–3,5). Ibandronate may be administered either in oral monthly doses or by intravenous bolus injection, with proven safety and effectiveness against placebo(1,2). The clinical follow-up is made with evaluation of ALP levels, the most sensitive marker for osteoblastic activity that must be performed every 3–6 months(1,2). Follow-up with CT and MRI can prove the pharmacological efficacy, as well as evaluating the possible complications of the disease itself, such as malignant degeneration or pathological fractures(1,4,5,8). The ill-defined, left-sided coxofemoral pain initially reported by the patient triggered a comprehensive diagnostic investigation. Such complaint, whose site did not coincide with the radiological findings, was later attributed to left-sided trochanteric bursitis that was managed with clinical symptomatic treatment and physiotherapy. Once the treatment for the bone disease was established, the patient did not complain of pain anymore. Finally, the authors emphasize that particular attention should be paid to male and elderly patients with pain in the lumbar spine and in the coxofemoral joint, because of the potential risk for development of neoplasias in this group of patients(4,5,8). The radiologist must be attentive to the possible presentations and complications of the disease, even in uncommon sites, trying whenever possible correlating them with the patients’ clinical symptoms. Acknowledgment To Dr. Marta Maria Chagas Medeiros, Associated Professor of Rheumatology at Universidade Federal do Ceará, for the revision and suggestions on the present study. REFERENCES 1. Griz L, Caldas G, Bandeira C, et al. Paget’s disease of bone. Arq Bras Endocrinol Metab. 2006;50:814–22. 2. Cundy T, Bolland M. Paget disease of bone. Trends Endocrinol Metab. 2008;19:246–53. 3. Dell’Atti C, Cassar-Pullicino VN, Lalam RK, et al. The spine in Paget’s disease. Skeletal Radiol. 2007;36:609–26. 4. Smith SE, Murphey MD, Motamedi K, et al. From the archives of the AFIP. Radiologic spectrum of Paget disease of bone and its complications with pathologic correlation. Radiographics. 2002;22:1191–216. 5. José FF, Pernambuco ACA, Amaral DT. Doença de Paget do osso. Einstein. 2008;6(Supl 1):S79–S88. 6. Love C, Din AS, Tomas MB, et al. Radionuclide bone imaging: an illustrative review. Radiographics. 2003;23:341–58. 7. Roberts MC, Kressel HY, Fallon MD, et al. Paget disease: MR imaging findings. Radiology. 1989;173:341–5. 8. Matiotti SB, Tramunt CS, Duarte RD, et al. Degeneração sarcomatosa de doença de Paget do calcâneo: relato de caso. Radiol Bras. 2009;42:63–5. 1. Master, Professor Assistant of the Discipline of Rheumatology, Course of Medicine, Universidade Estadual do Ceará (UECE), Fortaleza, CE, Brazil. 2. MD, Specialist in Radiology, Professor of the Discipline of Radiology, Course of Medicine, Universidade de Fortaleza (Unifor), Fortaleza, CE, Brazil. 3. Graduate Student of Medicine, Universidade Estadual do Ceará (UECE), Fortaleza, CE, Brazil. Mailing address: Dra. Fernanda Nogueira Holanda Ferreira Braga Coordenação de Medicina, Centro de Ciências da Saúde, Universidade Estadual do Ceará (UECE) Avenida Paranjana, 1700, Campus do Itaperi Fortaleza, CE, Brazil, 60740-000 Email: fnhfb@yahoo.com.br Received May 10, 2010 Accepted after revision July 23, 2010 Study developed at Universidade Estadual do Ceará (UECE), Fortaleza, CE, Brazil |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554