Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 5 - Sep. / Oct. of 2010
Vol. 43 nº 5 - Sep. / Oct. of 2010
|
ORIGINAL ARTICLE
|
|
Impact of health surveillance actions on the program of quality control in mammography in the Paraíba State, Brazil, from 1999 to 2003 |
|
|
Autho(rs): Maria Magdala de Brito Ramos1; Renato Dimenstein2; Henrique Manoel Lederman3 |
|
|
Keywords: Mammography; Quality control; Breast cancer; Health surveillance. |
|
|
Abstract: INTRODUCTION
Mammography is one of the most effective methods for the early diagnosis of malignant tumors. With this technology 85–95% of breast cancer can be detected in women above 50 years of age(1). The lack of effective mechanisms of primary prevention of breast cancer indicates the necessity of focusing efforts on the early detection of malignant lesions. In this context, epidemiological studies indicate that mammography still remains as the main strategy for the early detection of this disease(2). In Brazil, mortality and morbidity rates have changed over the last 40 years with a significant increase in the incidence of chronic-degenerative diseases, particularly breast cancer(3). According to data reported by Instituto Nacional de Câncer (National Cancer Institute), 49,240 new cases of breast cancer are estimated for 2010 in Brazil. In 2008, the number of deaths achieved 11.860 (11,375 women and 125 men), with an index of 40 new cases for every 100,000 women, which makes this neoplastic disease presentation the major cause of death among the different types of cancer affecting women. In the Paraíba State, 550 new cases of breast cancer are estimated for 2010(4). In the Unit II (HC III) of Hospital do Câncer in Rio de Janeiro, where many women present with mammograms previously performed in centers of the Brazilian unified health system (Sistema Único de Saúde – SUS/RJ), more than 70% of these imaging studies were rejected because of their poor quality and, consequently, had to be repeated at the HC III. The main causes for rejection were the following: set-up errors, poor contrast, artifacts and underdeveloped films(5). In a study evaluating images quality in mammography centers located in the Federal District, Corrêa et al.(6) have concluded that the main factor causing loss of quality in mammographic images was the performance of image processing machines. With a view to the relevance of the good image quality in mammography, the Ministry of Health and societies involving radiologists, particularly Colégio Brasileiro de Radiologia e Diagnóstico por Imagem – CBR (Brazilian College of Radiology and Imaging Diagnosis), have started recommending that mammography services undergo a continued program of quality certification implemented by the CBR itself. At the same time, state and municipal health surveillance agencies have adopted actions aimed at implementing their programs of quality control in mammography services, as a contribution for the early detection of breast cancer. The present study is aimed at evaluating the impact of health surveillance actions on the quality of mammograms in the Paraíba State. MATERIALS AND METHODS Image quality was evaluated in 17 mammography services in the Paraíba State during the period from 1999 to 2003, according to the Donabedian model(7), dividing the evaluation into the following categories: structure, processes and results. Structure and processes were evaluated according to the Guidelines of Visual Inspection for Radiodiagnosis Services proposed by the Agência Nacional de Vigilância Sanitária – Anvisa (Brazilian Agency of Health Surveillance). The results evaluation was focused on the images quality aimed at the early detection of breast cancer, and was based on criteria described on Table 1, according to the technical requirements of the Ministry of Health Ordinance No. 453/98(8), the European Guidelines for Quality Assurance in Mammography Screening (9) and the CBR guidelines. 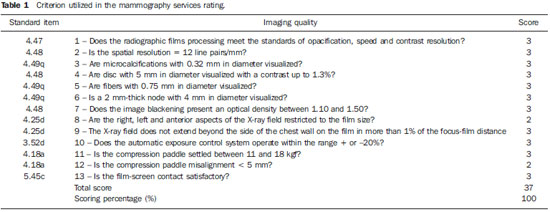 Initially, the institutions were registered, and data on their equipment and materials were collected, with subsequent testing based on technical parameters established by the Ministry of Health Ordinance No. 453/98, as well as by the CBR certification program. The tests covered the following topics: X-ray beam collimation, performance of the automatic exposure control, breast compression force, compression paddle alignment, cassette integrity testing, and processing quality. The tests results will be scored according to criteria on Table 1 and presented year by year on Figures 1, 2, 3 and 4. 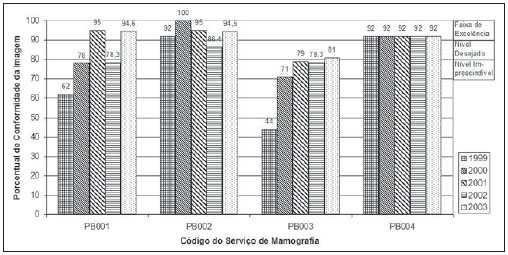 Figure 1. Evaluation of imaging quality in mammography services of the Paraíba State in the period 1999–2003 (PB001, PB002, PB003 and PB004). 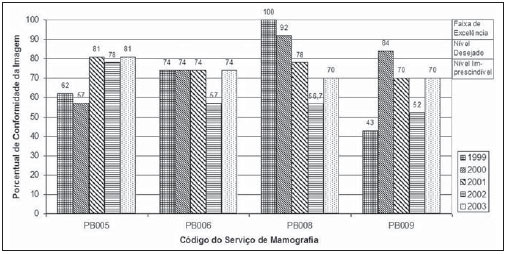 Figure 2. Evaluation of imaging quality in mammography services of the Paraíba State in the period 1999–2003 (PB005, PB006, PB008 and PB009). 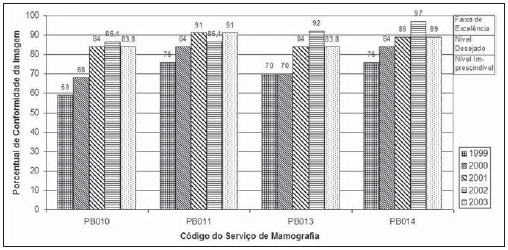 Figure 3. Evaluation of imaging quality in mammography services of the Paraíba State in the period 1999–2003 (PB010, PB011, PB013 and PB014). 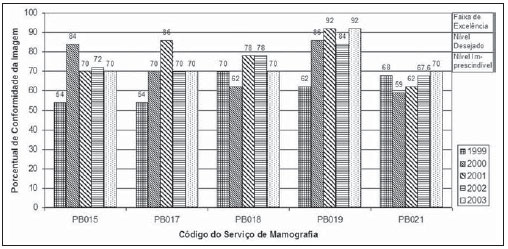 Figure 4. Evaluation of imaging quality in mammography services of the Paraíba State in the period 1999–2003 (PB015, PB017, PB018, PB019 and PB021). The evaluation of mammography services was based on the scoring of quality requirements for radiological breast images established by the Ordinance No. 453/98 and presented on Table 1. Figures 1, 2, 3 and 4 show the mammography services identified by their respective codes PB XX, where X represents the number corresponding to the service, and the bars correspond to the scoring achieved by the services at each year of evaluation. Figure 5 represents the mean rate of image quality for all the services as a whole year by year. 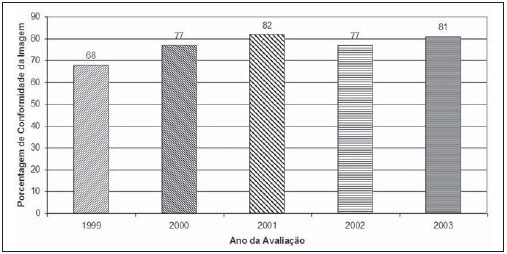 Figure 5. Mean rate of image quality in mammography services of the Paraíba State in the period 1999–2003. Aiming at obtaining a quantitative method to follow-up the improvement of the services, rating levels were established as follows: – Excellence range: > 90%. – Desired level: > 80% and < 90%. – Minimum rating level: > 70% and < 80%. – Undesired level: < 70%. RESULTS The quality results achieved for 1999 indicate that only five mammography services (29%) could produce images with satisfactory quality at the minimum mandatory rating level (Table 2). In 1999, the mean scoring for the mammography services evaluated achieved 68%; In 2000, at the second evaluation of images quality, the mean appropriateness level achieved 77%; in 2001 (the third year of evaluation), the mean appropriateness achieved 82%; in 2002 (the fourth year), 77%; in 2003, 81%. As regards the quantitative rating, the comparative results obtained for the mammography services in operation between 1999(11) and 2003(12) are shown on Figures 1, 2, 3 and 4. DISCUSSION The present study demonstrates peculiarities that have played a key role in the achievement of such results. One of these peculiarities was the fact that, in the Paraíba State, the health surveillance is done by an agency, which gives the State Health Surveillance Agency (Agência Estadual de Vigilância Sanitária – Agevisa) absolute administrative and financial independence and, consequently, allowing greater autonomy. Other crucial factors were the following: the formalization of technical cooperation agreements with CBR and Instituto de Radioproteção e Dosimetria (IRD/CNEN), and the publication of the Ordinance No. 453/1998, determining requirements regarding quality control testing as well as radioprotection of professionals, patients and general public in radiological centers. According Hendrick et al.(10), about 47% of mammography centers are not approved in the American College of Radiology (ACR) accreditation program, with shortcomings related to the performance of image processing machines. Notwithstanding the difference between the protocol for accreditation by the ACR and the one utilized in the present methodological format, the issue to be discussed is the necessity of a periodical and recurrent evaluation of quality. Testing in mammography centers represents only a momentary analysis of the imaging system performance. The issue to be considered is the frequency of actions aimed at maintaining the imaging quality. Therefore, the discussion implied in the present investigation is the histogram of compliance parameters, as shown on Figure 5, demonstrating a considerable progress in the imaging quality along the period of 1999 (68%), 2000 (77%), 2001 (82%), 2002 (77%) and finally 2003 (81%)(11,12). This progression indicates that, on average, the mammography services operating in the Paraíba State have achieved a compliance level for imaging quality above 80%, a level considered as desirable in order to provide the female population with goodquality assistance for early detection of breast cancer. On the chart shown on Figure 1, one can also observe that, in 2002 a subtle decrease occurred in the level of quality as compared with the previous year, justified by the inclusion of new requirements in the program, year by year, such as the obligatoriness of the monthly presentation of breast phantom images by the mammography services. As a rule, the loss of the imaging quality in mammography and the consequential reduction in the diagnostic capacity are not caused by a single factor. Most of times, a summation of inappropriate adjustments regarding images processing, patients’ positioning, automatic mammographic exposure systems, etc., significantly degrades the imaging quality. Therefore, the establishment of excellence criteria allows the evaluation of these factors as whole in order to achieve an accurate imaging diagnosis. In spite of the fact that the present study has not contemplated all the parameter for equipment evaluation, such as entrance skin dose, half-value layer, among others, the excellence criteria adopted in the present study has allowed the establishment of qualitative and evolutive parameters for mammography services, filling a gap in the current Anvisa standards and in the Ordinance No. 453/98 which recommend quality control testing but lack specification of scoring criteria to guide the actions of health surveillance. Considering that the objective of the present study was establishing quantitative criteria for evaluating mammography services, the authors utilized the parameters established by Anvisa. However, from the view of the supervisory body, the set of criteria adopted as a reference, as individually analyzed, not always depicts the total quality of the services, i.e., the final imaging quality standard. Therefore, such set of criteria is not sufficient to determine a compliance level regarding the presence of acceptable performances to authorize, or not, the operation of a mammography service. This situation becomes critical because a greatest majority of the mammography services included in the present study demonstrated non-compliant performance in some images processing and/or acquisition parameters, independently from the results in terms of diagnosis. Based on the present results, the authors could observe that, in addition to the values defined on the Ordinance No. 453/98, it is necessary to establish excellence criteria and levels, to allow the adoption of a supervisory body policy in the process of inspection and interdiction of mammography services. The duality between inspection actions and quality testing can be discussed in terms of the process of images acquisition itself. This statement is supported by the present discussion, based on the fact that in the sampling of data regarding the mammography services, despite the inappropriateness in relation to processing parameters, besides the established limits as compared with the breast phantom images, the quality was considered as satisfactory in terms of structures visualization. Therefore, in these cases, the key issue is to define the criteria to be adopted by the supervisory body in terms of health promotion. Small variations in the processing levels justify, or not, the adoption of legal measures against mammography services. Inappropriate parameters justify health surveillance actions, provided the compliance data resulting from the tests be analyzed in conjunction with excellence criteria in order to indicate if the mammography service remains within acceptable performance standards along time. The results of the present study point out the joint responsibility of mammography services, supervisory bodies, technical assistance companies and the medical society aiming at a continuous improvement of mammographic images at the level of an early diagnosis, which requires the adoption of a strategy involving the implementation of quality control, social responsibility and certification programs. CONCLUSION The technical actions adopted in the 17 mammography services evaluated in the Paraíba State in compliance with the health surveillance standards and actions between 1999 and 2003 have led the authors to conclude that, after the implementation of specific quality control programs a positive impact was observed in relation to the improvement of mammographic images quality. REFERENCES 1. Morrison AS. Screening in chronic disease. 2nd ed. New York, NY: Oxford University Press; 1992. 2. Pereira MG. Serviços de saúde. In: Pereira MG. Epidemiologia – teoria e prática. Rio de Janeiro, RJ: Guanabara Koogan; 2000. p. 513–37. 3. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Instituto Nacional de Câncer. Coordenação de Prevenção e Vigilância. Atlas de mortalidade por câncer no Brasil, 1979-1999. Rio de Janeiro, RJ: INCA; 2002. 4. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Instituto Nacional de Câncer. Coordenação de Prevenção e Vigilância. Estimativas da incidência e mortalidade por câncer no Brasil, 2006. Rio de Janeiro, RJ: INCA; 2005. 5. Canella EO, Kestelman FP, Barbalho TMM, et al. Causas de mamografias rejeitadas no Hospital do Câncer III. Rev Imagem. 2004;26(Supl 1):96 [tema livre 07.03]. 6. Corrêa RS, Peixoto JE, Silver LD, et al. Impacto de um programa de avaliação da qualidade da imagem nos serviços de mamografia do Distrito Federal. Radiol Bras. 2008;41:109–14. 7. Donabedian A. Explorations in quality assessment and monitoring. Vol. 1, The definition of quality and approaches to its assessment. Ann Arbor, MI: Health Administration Press; 1980. 8. Brasil. Secretária de Vigilância Sanitária. Ministério da Saúde. Diretrizes de proteção radiológica em radiodiagnóstico médico e odontológico. Portaria n° 453. Brasília, DF: Diário Oficial da União, 1/6/1998. 9. Perry N, Broeders M, de Wolf C, et al. European guidelines for quality assurance in mammography screening. 3rd ed. Luxembourg: Office for Official Publications of the European Communities; 2001. 10. Hendrick RE, Smith RA, Wilcox PA. ACR accreditation and legislative issues in mammography. In: Haus AG, Yaffe MJ, editors. Syllabus: a categorical course in physics, technical aspects of breast imaging. Oak Brook, IL: Radiological Society of North America; 1993. p. 137–49. 11. Paraíba. Agência Estadual de Vigilância Sanitária. Relatório de atividades anual – 1999. João pessoa, PB: Agevisa. 12. Paraíba. Agência Estadual de Vigilância Sanitária. Relatório de atividades anual – 2003. 1. Master, Fellow PhD degree at Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. 2. Master, Responsible for the area of Physics Applied to Nuclear and Radiological Medicine, Hospital Albert Einstein, São Paulo, SP, Brazil. 3. Titular Professor, Department of Imaging Diagnosis, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. Mailing address: Dra. Maria Magdala de Brito Ramos Rua Marquês de Itu, 797, ap. 111, Vila Buarque São Paulo, SP, Brazil, 01223-001 E-mail magdalaramos@yahoo.com.br Received March 8, 2010 Accepted after revision June 11, 2010 Study developed at Agência Estadual de Vigilância Sanitária do Estado da Paraíba (Agevisa), João Pessoa, PB, Brazil |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554