Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 5 - Sep. / Oct. of 2010
Vol. 43 nº 5 - Sep. / Oct. of 2010
|
ORIGINAL ARTICLE
|
|
Adjuvant radiotherapy in high-grade extremity sarcomas |
|
|
Autho(rs): Carlos Antônio da Silva Franca1; Felipe José Cordeiro Queiroz Marques2; Antonio Carlos Pires Carvalho3; Antonio Belmiro Rodrigues Campbell Penna4; Sérgio Lannes Vieira5 |
|
|
Keywords: Sarcoma; Radiotherapy; Brachytherapy; Chemotherapy; Conservative treatment. |
|
|
Abstract: INTRODUCTION
Soft tissue sarcomas are rare tumors, representing approximately 1% of malignant neoplasias in adults. Men are more frequently affected than women, and black individuals are more affected than the white ones(1). In Brazil, data from 1997 demonstrate an incidence of 2.8 cases per 100,000 inhabitants(2). Sarcomas comprise a very heterogeneous group of tumors with high malignancy characteristics and high risk for local or distant recurrence(3). The treatment of sarcomas is still a challenge, requiring multidisciplinary approach with the intent of achieving complete tumor resection and appropriate organ functioning. However, local failures of about 10% to 20% still occur(2). Even with all progress achieved over the past decades, controversies on the best treatment approaches still remain, such as the type of surgery (either amputation or conservative resection) and either neoadjuvant or adjuvant therapy, radiotherapy (pre- versus post-surgical, type, dose, fraction), and chemotherapy(4). External beam radiotherapy (EBRT) has been utilized in an attempt to achieve greater local management of sarcomas, however, there is a significant increase of late toxicity associated with high-dose EBRT, a treatment that is necessary for a satisfactory local management of complications such as tissue fibrosis, loss of articular function, neuritis and limb edema(5). Brachytherapy has emerged as an attractive treatment modality considering the possibility of reducing local complications without reducing the dose applied on the tumor bed(6). The objective of the present study was to evaluate the therapies utilized in the author’s institution for treatment of highgrade extremity sarcomas, considering the global survival rates achieved with the multidisciplinary treatment. MATERIALS AND METHODS Retrospective study of patients with diagnosis of sarcoma treated at the Service of Radiation Oncology in the period from 1993 to 2007. The present study evaluated 36 adult patients staged by the classification developed by the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC)(7), as follows: T2a – tumors > 5 cm in its greater dimension, superficial (exclusively located above the superficial fascia, without invading it); T2b – tumors > 5 cm in its greater dimension, deeply located (under the superficial fascia, invading or penetrating it). The tumors were classified into IIb (T2a high-grade N0 M0) and III (T2b high-grade N0 M0). All patients were selected according to the following inclusion criteria:
At the institution’s service, preference is given to treatment with adjuvant external beam radiotherapy, whenever possible utilizing a dose boost means of high-dose rate brachytherapy, with intraoperative implantation of catheters in order to reduce the external radiation dose and the possibility of late fibrosis(8). Techniques description EBRT – Radiotherapy must be compartmental and an appropriate demarcation of irradiation fields is extremely important, and it should always be based on radiological images, with description of margins and placement of intraoperative marking clips, exceeding the fields by 5 cm longitudinally and by 2 cm in the laterally and at depth. Individual molds are made to allow the correct positioning and treatment reproducibility. During the technical planning, one endeavors to preserve the adjacent healthy tissues as much as possible, and in the extremities, to avoid irradiating their whole circumference in order to minimize the possibility of edema and fibrosis. The doses utilized for EBRT may range from 40 to 60 Gy, with daily fractioning of 1.8 to 2 Gy, but in cases of doses > 50 Gy with extensive fields and no possibility brachytherapy boost, a boost dose with EBRT at 10 Gy is utilized with reduced fields, considering a 2 cm-margin beyond the marking clips. High-dose rate brachytherapy – Brachytherapy allows the restrict irradiation of the surgical bed, with high-dose rate in an attempt to improve local management and reducing complications. Brachytherapy is performed after removal of the surgical specimen, and subsequent demarcation of the tumor bed with an open surgical field, placing the catheters with 2 cm spacing from each other, implanting them as parallel as possible, at 2 cm beyond the marking clips. The treatment is initiated 5 days after surgery, on an outpatient basis, with two daily applications at a minimum six-hour interval. The dose may range from 16.2 to 35 Gy, with fractions of 2.7 to 3.5 Gy. The following treatment schemes were utilized:
Statistical analysis For the purposes of data analysis, central trend and scatter measurements were calculated, with the Student’s t-test being utilized for continuous variables, and the ?2 test for categorical variables, with the adoption of a significance level of 5% (p ? 0.05) and 95% confidence interval of (CI 95%). The actuarial 7-year disease-free survival was analyzed by means of the Kaplan-Meier method and by the log-rank test. The statistical analysis was carried out by means of the Statistical Package for Social Sciences (SPSS) for Windows, version 13 (SPSS Inc®; Chicago, IL, USA). RESULTS The present study evaluated 36 patients who met the above mentioned inclusion criteria. All the patients were submitted to surgical treatment followed by adjuvant EBRT, with 4 of them (11%) receiving high-dose rate brachytherapy boost, and 7 (19%) receiving chemotherapy, all because of positive surgical margin. Two patients (5.5%) were staged as IIb and the other 34 (94.5%), as stage III. All the 36 patients presented with high histological grade tumors. The mean age of the patients was 53 years (18–92/CI 95%: 48–61), and 14 (39%) were men and 22 (61%), women. Disease-free surgical margins were found in 29 patients (81%) and positive margins in 7 patients (19%). The mean EBRT dose was 50 Gy (30– 61 Gy/CI 95%: 47–53 Gy), with brachytherapy boost being performed in four patients because of the presence of the specialist in radiation oncology in the surgical act for catheters implantation, with doses ranging from 16.5 to 35 Gy according to the previously described treatment schemes. Chemotherapy was indicated in seven cases (19%) with positive margins, being utilized simultaneously with radiotherapy (five patients; 71%) or two to four months following radiotherapy (two patients; 29%). The patients with positive margins did not receive brachytherapy. Fifteen patients presented with local and/or distant recurrence (42%), with a mean time for recurrence of 48 months (36–84/CI 95%: 45–65), and all of them died in a mean time of 72 months (24–96/CI 95%: 65–84). Twenty-one patients (68%) are currently with no clinical /radiological evidence of local or distant recurrence. The mean follow-up time is 88 months (24–180/CI 95%: 74–102). The actuarial global 7-year survival rate was 80% (Figure 1). 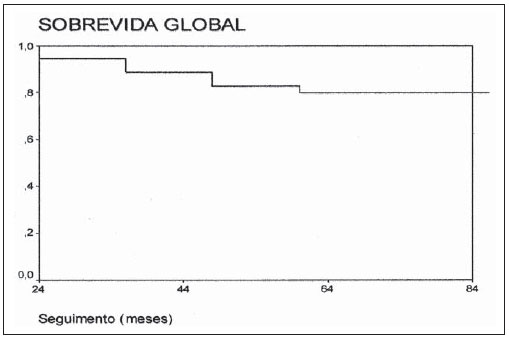 Figure 1. Global survival. Table 1 shows a comparison of data regarding living patients and those who progressed to death. The present study demonstrates that, among all the evaluated patients and those at high risk (high-grade) the worst prognosis was observed in the patients with positive margins (p = 0.013), and that it seems there is a difference between oldest patients and worst prognosis (p = 0.06), even without a statistically significant difference. Chemotherapy did not demonstrate to improve the prognosis in high-risk patients (with positive margins), and it should be object of further studies (p = 0.5). The radiotherapy dose has also demonstrated to be significant in the determination of the prognosis (p = 0.6) while brachytherapy seems to have been beneficial as it demonstrated to have achieved the management of the disease in all patients who were submitted to this treatment modality (p < 0.001). 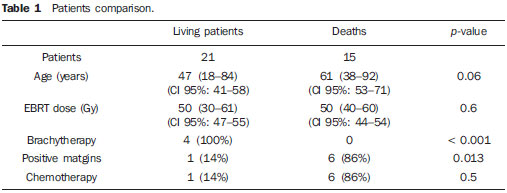 DISCUSSION In the present study, the analysis of the 36 patients presenting with extremity sarcomas demonstrated 80% global 7-year survival rate besides an association between worst prognosis and positive surgical margins. The association with highdose rate brachytherapy boost seems to improve the local management of the disease and the global survival in association with surgery and adjuvant EBRT(9,10). However, considering that in the present study, brachytherapy associated with EBRT was utilized in a small number of patients further studies with a higher number of patients are required to achieve conclusive data. Increase EBRT doses (around 40 to 70 Gy)(4,11), should be used with a view on improving local management and lower rates of distant recurrence. In the present study, the median dose was 50 Gy, most of times because of the advanced stage of the cases, the greatest majority being stage III, which resulted in extensive irradiation fields. The study developed by Trovik(12) on factors of worst prognosis has observed an association between older patients (> 50 years) with risk of metastasis, and decreased global survival rates in the group of older patients. In the present study, it seems there is an association between advanced ages and risk for death, with a great difference regarding median ages between surviving patients and those who died, even without a statistically significant association. In many cases recurrence onset seems to occur two or three years following the surgery (13); in the present study the median interval for recurrence was 48 months. Previous studies(14–16) have found strong association between positive surgical margins and decreased rates of systemic management, corroborating the results of the present study, which observed that positive surgical margins represent the most important factor for a worst prognosis. Beltrami et al.(4) have demonstrated rates of disease management in five and 10 years corresponding to 91.5% and 87%, respectively, with the use of surgery and EBRT. These rates are similar to the findings of the present study, in which the global 7-year survival rate was 80%. According to some authors(9,10), a more conservative surgery associated with highdose rate radiotherapy achieved by means of combined EBRT and brachytherapy, increases the local management of sarcomas in up to 80–90%. In the present study, such combination presented a rate of local management corresponding to 100%. However the number of patients submitted to such combined treatment was small, mostly because of the difficulty in contacting the surgical team. According to Laskar et al.(5), the use of brachytherapy, whether in association with EBRT or not, seems to be an effective modality in cases of sarcomas treated with limb conservative surgery, which was also observed in the present study, perhaps by the possibility of high irradiation doses with the combined treatment. The utilization of adjuvant chemotherapy in the treatment of sarcomas is quite controversial and, for some authors, it would decrease the disease management rates(17,18). In the present study, chemotherapy was utilized in patients with positive margins, a factor for worst prognosis, with disappointing results. However, more recent reviews have pointed to studies with new drugs and drugs aimed at histological subtypes, for example, imatinib, docetaxel, gemcitabine and pazopanib for cases of patients with high-grade or T2 sarcomas( 11,19), in which the responses have been encouraging, but still requiring further studies for the definition of a rational utilization of adjuvant and/or neoadjuvant chemotherapy in sarcomas, considering the treatment toxicity(11,19). CONCLUSION The use of conservative surgery in association with EBRT presents good disease management rates. Combined brachytherapy and EBRT increases radiation doses in the surgical bed, leading to better management rates. The use of chemotherapy still demands supplementary studies. In the present study, the authors could conclude that combined surgery + EBRT is an effective treatment for extremity sarcomas with excellent responses and improved global survival in the possibility of association with brachytherapy. REFERENCES 1. Muhic A, Hovgaard D, Mrrk Petersen M, et al. Local control and survival in patients with soft tissue sarcomas treated with limb sparing surgery in combination with interstitial brachytherapy and external radiation. Radiother Oncol. 2008;88:382–7. 2. Pellizon ACA, Salvajoli JV, Novaes PERS, et al. Cirurgia conservadora, radioterapia externa e reforço de dose com braquiterapia de alta taxa de dose: uma nova perspectiva no tratamento de sarcomas de partes moles do adulto. Radiol Bras. 2002;35:89–92. 3. Storm HH. Survival of adult patients with cancer of soft tissues or bone in Europe. Eur J Cancer. 1998;34:2212–7. 4. Beltrami G, Rüdiger HA, Mela MM, et al. Limb salvage surgery in combination with brachytherapy and external beam radiation for highgrade soft tissue sarcomas. Eur J Surg Oncol. 2008;34:811–6. 5. Laskar S, Bahl G, Puri A, et al. Perioperative interstitial brachytherapy for soft tissue sarcomas: prognostic factors and long-term results of 155 patients. Ann Surg Oncol. 2007;14:560–7. 6. Pisters PW, Harrison LB, Woodruff JM, et al. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J Clin Oncol. 1994;12:1150–5. 7. Brasil. Ministério da Saúde. TNM: classificação de tumores malignos. 6ª ed. Rio de Janeiro, RJ: INCA; 2004; p. 120–4. [acessado em: 2 de julho de 2010. Disponível em: http://www.inca.gov.br/tratamento/tnm/tnm2.pdf 8. Chun M, Kang S, Kim BS, et al. High dose rate interstitial brachytherapy in soft tissue sarcoma: technical aspects and results. Jpn J Clin Oncol. 2001;31:279–83. 9. Suit HD, Russell WO, Martin RG. Management of patients with sarcoma of soft tissue in an extremity. Cancer. 1973;31:1247–55. 10. Lindberg RD, Martin RG, Romsdahl MM. Surgery and postoperative radiotherapy in the treatment of soft tissue sarcomas in adults. Am J Roentgenol Radium Ther Nucl Med. 1975;123:123–9. 11. Pisters PW, O’Sullivan B, Maki RG. Evidencebased recommendations for local therapy for soft tissue sarcomas. J Clin Oncol. 2007;25:1003–8. 12. Trovik CS; Scandinavian Sarcoma Group Project. Local recurrence of soft tissue sarcoma. A Scandinavian Sarcoma Group Project. Acta Orthop Scand Suppl. 2001;72:1–27. 13. Gustafson P. Soft tissue sarcoma. Epidemiology and prognosis in 508 patients. Acta Orthop Scand Suppl. 1994;65:1–31. 14. Pisters PW, Harrison LB, Leung DH, et al. Longterm results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–68. 15. Gronchi A, Casali PG, Mariani L, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol. 2005;23:96–104. 16. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97:2530–43. 17. Antman KH. Adjuvant therapy of sarcomas of soft tissue. Semin Oncol. 1997;24:556–60. 18. Gherlinzoni F, Picci P, Bacci G, et al. Late results of a randomized trial for the treatment of soft tissue sarcomas (STS) of the extremities in adult patients. Proc Am Soc Clin Oncol. 1993;12:abstr 1633. 19. Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer – Soft Tissue and Bone Sarcoma Group (EORTC Study 62043). J Clin Oncol. 2009;27:3126–32. 1. MSc in Medicine, MD, Specialist in Radiation Oncology at Instituto Brasileiro de Oncologia (IBO) and at Pontifícia Universidade Católica do Rio de Janeiro (PUC-Rio), Rio de Janeiro, RJ, Brazil. 2. MD, Specialist in Radiation Oncology at Hospital São Vicente de Paulo (HSVP) and at Pontifícia Universidade Católica do Rio de Janeiro (PUC-Rio), Rio de Janeiro, RJ, Brazil. 3. PhD, MD, Radiologist at Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFFUFRJ), Rio de Janeiro, RJ, Brazil. 4. PhD, MD, Specialist in Radiation Oncology at Instituto Brasileiro de Oncologia (IBO) and at Pontifícia Universidade Católica do Rio de Janeiro (PUC-Rio), Rio de Janeiro, RJ, Brazil. 5. Full Professor, Course of Post-Graduation in Radiation Oncology – Pontifícia Universidade Católica do Rio de Janeiro (PUCRio), Head of the Service of Radiotherapy at Hospital São Vicente de Paulo (HSVP) and Instituto Brasileiro de Oncologia (IBO), Rio de Janeiro, RJ, Brazil. Mailing address: Dr. Carlos Antônio da Silva Franca Rua Presidente Pedreira, 27, Ingá Niterói, RJ, Brazil, 24210-470 Email: csfranca@ig.com.br Received March 17, 2010 Accepted after revision July 26, 2010 This study was performed by Hospital São Vicente de Paulo (HSVP), Rio de Janeiro, RJ, with the support of Instituto Brasileiro de Oncologia (IBO), Pontifícia Universidade Católica do Rio de Janeiro (PUC-Rio) and Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), Rio de Janeiro, RJ, Brazil |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554