Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 4 - July / Aug. of 2010
Vol. 43 nº 4 - July / Aug. of 2010
|
ORIGINAL ARTICLE
|
|
Accuracy in the reproducibility of daily patients' setup in 3D conformal radiotherapy for prostate cancer |
|
|
Autho(rs): Adelmo José Giordani1; Rodrigo Souza Dias2; Helena Regina Comodo Segreto3; Roberto Araujo Segreto4 |
|
|
Keywords: Setup reproducibility; Prostate cancer; Conformal radiotherapy. |
|
|
Abstract: INTRODUCTION
One of the greatest challenges in radiotherapy is homogeneously delivering the prescribed radiation dose on a target volume and minimizing the radiation dose to the adjacent normal tissues(1-3). For such purpose, three-dimensional (3D) planning techniques with conformal radiotherapy with or without intensity modulation of the radiation beam (IMRT) have been utilized(4). Conformal radiotherapy can be considered as the standard treatment for localized prostate tumors, allowing the delivery of a higher radiation dose than conventional radiotherapy and a reduction of 40% to 50% in the irradiated volume of normal tissue(5). However, accuracy is required in the positioning of the patient and its reproducibility is indispensable, as any geometrical alteration may compromise the effectiveness of the treatment and increase the incidence of undesirable effects(6,7). As regards radiotherapy for prostate cancer, the definition of the target volume is fundamental for the planning and execution of the treatment. Usually, a margin is added to the tumor volume (gross tumor volume - GTV), for inclusion of the subclinical disease in the radiation field, which constitutes the clinical target volume (CTV). Additionally, a margin for geometric uncertainties including setup errors and organ motion is added, comprising the planning target volume (PTV)(8). The size of the margins depends on the magnitude of the uncertainties, and in the specific case of prostate treatment, margins of 10 mm from the CTV to PTV are considered as standard. There may be a reduction of 6 to 8 mm in the posterior margin towards the rectum(8). Such values, however, are given as guidance, and each institution should decide upon the most appropriate margin(9). It is important to highlight that intensity modulated radiotherapy allows the achievement of isodose curves more restricted to the PTV, which generates even greater preoccupations with the patients' setup(4). The capability of reducing the margins from CTV to PTV is related to the decrease in setup errors, which can be attained through the setup correction based on bone references on radiological images obtained during the treatment(10). Because of the importance of reproducing in the linear accelerator what was previously performed in the simulation, and because of the need to concentrate the dose in a restricted target volume, techniques have been developed for checking the positioning of the field to be treated, before and during the treatment(11-13). For the verification of geometric uncertainties, during treatment the patients are submitted to radiography whose images are compared with digitally reconstructed radiography (DRR) images obtained from the planning tomography. Electronic portal images (EPI) may also be used for the same purpose, besides allowing corrections during treatment and reduction of errors from 5 mm to 3 mm(8). Studies suggest the performance of one to three computed tomography (CT) scans during treatment, to identify the organs motion. Magnetic resonance imaging (MRI) has also been utilized in treatment planning by means of computed planning systems that perform the images fusion, thus providing a better delimitation of the target volume. MRI offers greater advantages when compared with CT, as it provides a better visualization of the prostate, seminal vesicles, rectum and muscles(14). The use of fiducial markers has also been increasingly indicated, particularly for image-guided radiation therapy (IGRT). Thus, as a routine, one can reduce the PTV margins and, consequently, minimize doses to the rectum and bladder and still correct the patient's setup during the treatment(15). With developments in technology and the utilization of increasingly complex techniques, a higher accuracy in the determination and attainment of PTV is desirable. Patients' motion, setup errors and organs motion contribute to uncertainties in the treatment(9). The literature demonstrates that when patient's setup protocols and standardization of the process for checking such protocols are utilized, errors between 2 and 4 mm are observed in approximately 80% of the portal images(16). As regards the errors, the most significant are the systematic ones, as they lead to delivery of inappropriate doses to the target volume and to healthy organs. Random errors present lesser impact of the doses on the mentioned structures(4). In order to reduce such errors, several studies have suggested the daily evaluation of the fields reproducibility(2,6,11,12,17). Such process comprises two stages: the identification of the setup deviation and the decision on intervening in case such deviation is significant(2,12). The immobilization of the patient, in association with skin markings and alignment with the in-room laser, greatly reduces the uncertainties in the treatment(6). Even with all the above described resources and methods, it is still extremely difficult to eliminate all the uncertainties, and in order to significantly reduce them, protocols for checking setup errors and their correction have been created. Thus, the present study aims at evaluating the reproducibility of patients' setup in 3D conformal radiotherapy for prostate cancer, by utilizing digital planning, DRR and radiological films, in the anteroposterior and latero-lateral fields. MATERIALS AND METHODS Casuistics The present study included 120 patients with prostate tumors referred for routine radiotherapy. For the prostate planning, the patients were initially submitted to simulation with an Acuity (Varian Medical Systems; Palo Alto, CA, USA) apparatus. The patients were placed in dorsal decubitus, with fixed ankles and with the hands on the chest. The isocenter was located by means of an anterior radiographic view with a 10 × 10cm field, centered on the midline line of the patient's body and lower limit on the inferior border of the pubis, and a lateral view with the anterior limit of the field between 1.0 and 1.5cm posteriorly to the border of the pubis. These points were externally marked on the patient's skin. These same setup conditions were applied to tomography and to the 6 MV linear accelerator (Varian Medical Systems; Palo Alto, CA, USA). The tomographic images were processed in the computed planning system Eclipse (Varian Medical Systems; Palo Alto, CA, USA). Subsequently, the CTV and PTV were defined, the latter being the CTV with the addition of a 10mm margin, except for the posterior margin, which was of 8mm. As regards the treatment, all the patients received a dose > 72 Gy in one or two phases, depending on the disease staging. In all the patients, six entry portals were utilized. After the PTV definition, a new isocenter was delimited and a localization plane with 10 × 10cm fields at 0º, 90º and 270º was built (Figure 1). DRRs were then obtained from these fields, and the images were transferred to the simulator and served as a reference for the definition of the new isocenter on the patient, and such isocenter checking during treatment. 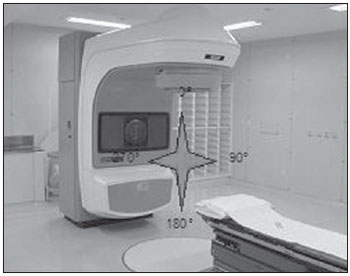 Figure 1. Angles definition. Anterior and lateral radiographic views on the 10 × 10cm field were performed on the first day of treatment and weekly thereafter. Such images were compared with the DRRs of the computed planning system. Values for the latero-lateral, cranio-caudal and antero-posterior displacements were obtained. Random evaluations were performed on 480 pairs of films (anterior and lateral views, with a total of 960 radiological images). Bone references were utilized in the evaluation of the images. In the anterior views, the distances from the isocenter to the true pelvis, and from the isocenter to the pectineal line were measured on the craniocaudal view and on the latero-lateral view, respectively (Figures 2A and 2B). Two measurements were performed on the lateral view: the distances from the isocenter to the pubic symphysis and to the sacrum (Figures 2C and 2D). 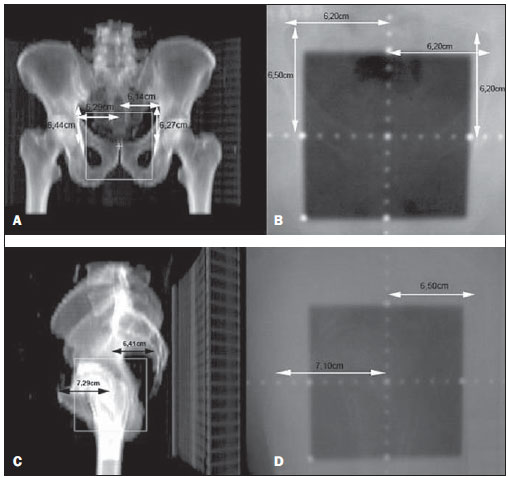 Figure 2. On A and B, anterior views of DRR and radiography are observed, and on C and D, the lateral views, respectively. The acceptance limit for positioning displacement was 2.5mm, established as standard error (SE = 2.5mm). For variations between 2.5 and 5mm, the correction was established in the linear accelerator, and when above 5mm, the patient was returned to the simulator for correction. Statistical analysis Excel for Windows version 2009 was utilized for descriptive analysis and calculation of means and standard deviations of the setup errors variations. RESULTS The results of the present study demonstrate that, at the anterior radiographic view (craniocaudal), 115 patients (95.8%) were within the standard error (2.5mm), and only 5 patients (4.2%) required corrections. As regards the latero-lateral view, 98 patients (81.67%) were within the acceptance limits, 21(17.5%) patients required corrections and only 1 patient (0.83%) returned to the simulator for re-planning. As regards the lateral view (antero-posterior), 102 patients (85%) presented setup variations < 2.5mm, 17(14.17%) patients required corrections and only 1(0.83%) patient returned to the simulator for replanning (Figure 3). 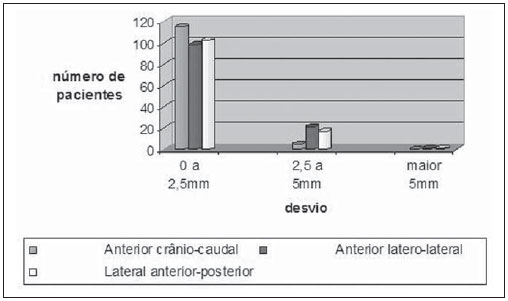 Figure 3. Positioning deviation versus number of patients. In the analysis of the anterior and lateral views of all the patients, 87.5% were within the standard error, 11.95% were between 2.5 and 5mm deviations and 0.55% above 5mm deviation. In the analysis of means and standard deviations regarding setup variations, one observed the values of 1.99 ± 1.25mm, 1.37 ± 0.84mm and 1.94 ± 1.10mm in the craniocaudal, latero-lateral and anteroposterior views, respectively. With respect to the application of the same distance evaluation system using bone references, Figure 4 shows anterior and lateral radiographic views with setup errors. 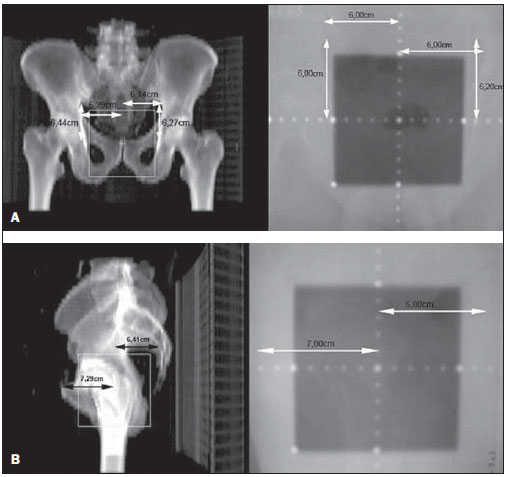 Figure 4. Radiographic image considered as inappropriate in relation to patient’s setup (A) on the anterior view, and (B) lateral view as compared with DRRs obtained in the planning system. DISCUSSION Protocols based on the literature and appropriate to the needs of the Unit of Radiotherapy of Universidade Federal de São Paulo were adopted in the present study, whose main purpose was establishing discipline and routine in the control of quality of radiotherapy planning for patients with prostate cancer in a public institution. Digital planning, DRR and radiological films were utilized in successiveevaluations of the reproducibility of patients' setup in 3D conformal radiotherapy for prostate cancer. The need for daily or periodical checking of the target localization in the treatment of prostate cancer with external radiation beam is controversial. The daily image-guided alignment (IGRT) is considered as the gold standard, however the costs and room time required are greater as compared with the ones required by conformal radiotherapy and IMRT(18). Displacement of the target volume corresponded to 1.99 ± 1.25mm and 1.37 ± 0.84mm on the craniocaudal and latero-lateral views, and 1.94 ± 1.10mm on the anteroposterior view. In the case of the prostate, variations in setup between 1.0 and 3.8mm on the anteroposterior field and from 1.2 to 3.5mm on the lateral field are reported when legs immobilizer devices are utilized(19). Setup variations setup observed with the use of markers directly implanted on the prostate allow the actual evaluation of the treatment volume, besides the attainment of an appropriate setup(20). Reduction in setup errors from 3.2mm to 1.4mm with a mean of 2.2 mm is obtained by instructing the patients to keep the bladder full in the moment of the treatment, besides the use of standardized rectal and fiducial markers in the prostate for all the patients(21). Considering the setup error values obtained in the present study, and by adding to these values the internal prostate motion which, according to the literature, presents variations between 1.8 and 5.8 mm anteroposteriorly, and of 1.4 to 3.3 mm superoinferiorly, the authors believe that a 10 mm margin would be enough to cover the setup error and the internal prostate motion(22). As shown in the literature, setup errors of 7.3mm anteroposteriorly, and 3 mm laterally may occur with the utilization of body immobilizer devices and actual delimitation of the treatment volume(23). In another study, setup variations of 7 to 10mm anteroposteriorly, and 4 to 6mm laterally were observed(24). Dose variations from 2.5% to 10% are observed when in vivo dosimetry is performed(25). The implementation of planning, simulation and treatment techniques is useful in the evaluation of patients' positioning displacement and establishes an error limit of 3 mm. Such limit should be used to assure the appropriate dose delivery to the target volume during conformal radiotherapy(24,26,27). Large displacements of the irradiated pelvic volumes must be corrected, in order to assure an appropriate dose distribution in the target volume and at the same time maintain the dose to healthy adjacent tissues within the tolerance parameters(28). Several authors have observed errors of up to 10mm in pelvic treatments, and recommend a new setup checking based on portal films in the simulator(29,30). The treatment effectiveness depends upon the minimization of setup errors and organs motion. Setup errors of up to 2 mm do not cause significant changes in the dose distribution on the CTV and rectal wall(31). Recent studies with frequent patients' setup checking and careful evaluation of the target volume margins show better dose distribution on this volume, providing better management of the disease and lower incidence of adverse effects(21,32). The satisfactory results achieved with conformal radiotherapy for different anatomical sites have increased the interest on this technique and stimulated the development of setup protocols and checkings. The data resulting from the comparison between radiological films and digitally reconstructed images demonstrate an appropriate reproducibility of patients' setup in conformal radiotherapy for prostate cancer and allow the establishment of an internal treatment quality control. REFERENCES 1. Stryker JA, Shafer J, Beatty RE. Assessment of accuracy of daily set-ups in prostate radiotherapy using electronic imaging. Br J Radiol. 1999;72:579-83. 2. Gilhuijs KG, Herk M. Automatic on-line inspection of patient setup in radiation therapy using digital portal images. Med Phys. 1993;20:667-77. 3. Kijewski P. Three-dimensional treatment planning. In: Mauch PM, Loeffler JS, editors. Radiation oncology: biology and technology. Philadelphia, PA: WB Saunders; 1994. p.10-33. 4. Zhu SY, Mizowaki T, Norihisa Y, et al. Comparisons of the impact of systematic uncertainties in patient setup an prostate motion on doses to the target among different plans for definitive external-beam radiotherapy for prostate cancer. Int J Clin Oncol. 2008;13:54-61. 5. Stanley S, Griffiths S, Sydes MR, et al. Accuracy and reproducibility of conformal radiotherapy using data from a randomised controlled trial of conformal radiotherapy in prostate cancer (MRC RT01, ISRCTN47772397). Clin Oncol (R Coll Radiol). 2008;20:582-90. 6. Alasti H, Petric MP, Catton CN, et al. Portal imaging for evaluation of daily on-line setup errors and off-line organ motion during conformal irradiation of carcinoma of the prostate. Int J Oncol Biol Phys. 2001;49:869-84. 7. Langmack KA. Portal imaging. Br J Radiol. 2001;74:789-804. 8. McGarry CK, Cosgrove VP, Fleming VAL, et al. An analysis of geometric uncertainty calculations for prostate radiotherapy in clinical practice. Br J Radiol. 2009;82:140-7. 9. Alonso-Arrizabalaga S, Brualla González L, Roselló Ferrando JV, et al. Prostate planning treatment volume margin calculation based on the ExacTrac X-Ray 6D image-guided system: margins for various clinical implementations. Int J Radiat Oncol Biol Phys. 2007;69:936-43. 10. Middleton M, See A, Rolfo A, et al. Intraprostatic fiducials for image guidance: workflow implications in a single linac department. Radiography. 2008;14:312-7. 11. Greer PB, Jose CC, Matthews JHL. Set-up variation of patients treated with radiotherapy to the prostate measured with an electronic portal imaging device. Australas Radiol. 1998;42:207-12. 12. Bollet MA, McNair HA, Hansen VN, et al. Can digitally reconstructed radiographs (DRRs) replace simulation films in prostate cancer conformal radiotherapy? Int J Radiat Oncol Biol Phys. 2003;57:1122-30. 13. Washington CM, Leaver TD, Myles J, et al. Simulator design and operation. In: Washington CM, Leaver DT, editors. Principles and practice of radiation therapy: practical applications. St. Louis, MO: Mosby; 1996. p.81-105. 14. Nakamura K, Shioyama Y, Tokumaru S, et al. Variation of clinical target volume definition among Japanese radiation oncologists in external beam radiotherapy for prostate cancer. Jpn J Clin Oncol. 2008;38:275-80. 15. Lock M, Catton C. High-precision radiotherapy: where are we going and how do we get there? Can J Urol. 2006;13 Suppl 2:34-6. 16. de Boer HCJ, Heijmen BJM. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys. 2001;50:1350-65. 17. Varian Medical System. Varian Portal Vision Match Guide, Therapists manual 2000. [cited 2009 Jun 10]. Available from: www.varian.com 18. Kupelian PA, Lee C, Langen KM, et al. Evaluation of image-guidance strategies in the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1151-7. 19. Hurkmans CW, Remeijer P, Lebesque JV, et al. Set-up verification using portal imaging: review of current clinical practice. Radiother Oncol. 2001;58:105-20. 20. Halperin R, Roa W, Field M, et al. Setup reproducibility in radiation therapy for lung cancer: a comparison between T-bar and expanded foam immobilization devices. Int J Radiat Oncol Biol Phys. 1999;43:211-6. 21. Lattanzi J, McNeely S, Hanlon A, et al. Daily CT localization for correcting portal errors in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:1079-86. 22. Rabinowitz I, Broomberg J, Goitein M, et al. Accuracy of radiation field alignment in clinical practice. Int J Radiat Oncol Biol Phys. 1985;11:1857-67. 23.Wu J, Haycocks T, Alasti H, et al. Positioning errors and prostate motion during conformal prostate radiotherapy using on-line isocentre set-up verification and implanted prostate markers. Radiother Oncol. 2001;61:127-33. 24. McGary JE, Grant W 3rd. A clinical evaluation of setup errors for a prostate immobilization system. J Appl Clin Med Phys. 2000;1:138-47. 25. Lanson JH, Essers M, Meijer GJ, et al. In vivo dosimetry during conformal radiotherapy requirements for and findings of a routine procedure. Radiother Oncol. 1999;52:51-9. 26. Mubata CD, Bidmead AM, Ellingham LM, et al. Portal imaging protocol for radical dose-escalated radiotherapy treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1998;40:221-31. 27. Wratten CR, Denham JW, Kron T, et al. 'When measurements mean action' decision models for portal image review to eliminate systematic setup errors. Australas Radiol. 2004;48:272-9. 28. Wyman DR, Ostapiak OZ, Gamble LM. Analysis of mechanical sources of patient alignment errors in radiation therapy. Med Phys. 2002;29:2698-704. 29. Morgan TL, Banks DA, Kagan AR. Radiation therapy port films: a quality assurance study. Int J Radiat Oncol Biol Phys. 1998;42:223-7. 30. Creutzberg CL, Althof VGM, de Hoog M, et al. A quality control study of the accuracy of patient positioning in irradiation of pelvic fields. Int J Radiat Oncol Biol Phys. 1996;34:697-708. 31. Noel C, Parikh PJ, Roy M, et al. Prediction of intrafraction prostate motion: accuracy of pre- and post-treatment imaging and intermittent imaging. Int J Radiat Oncol Biol Phys. 2009;73:692-8. 32. Verhey LJ, Goitein M, McNulty P, et al. Precise positioning of patients for radiation therapy. Int J Radiat Oncol Biol Phys. 1982;8:289-94. 1. Doctor Professor, Physicist responsible for the Unit of Radiotherapy, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil 2. Master, Physician Assistant at the Unit of Radiotherapy, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil 3. Post-Doctorate, Associate Professor, Department of Clinical and Experimental Oncology, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil 4. Private Docent, Associate Professor and Head for the Unit of Radiotherapy, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil Study developed at the Unit of Radiotherapy, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil Mailing address: Dr. Adelmo José Giordani Rua do Estilo Barroco, 708, ap. 91, Alto da Boa Vista 04709011, São Paulo, SP, Brazil E-mail: adelmogiordani@ig.com.br Received November 14, 2009 Accepted after revision May 7, 2010 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554