Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 5 - Sep. / Oct. of 2007
Vol. 40 nº 5 - Sep. / Oct. of 2007
|
ORIGINAL ARTICLE
|
|
Computed tomography in the evaluation of abdominal fat distribution associated with a hyperlipidic diet in previously undernourished rats |
|
|
Autho(rs): Carlos Alberto Soares da Costa, Erika Gomes Alves, Gabriele Paula Gonzalez, Thais Barcellos Côrtez Barbosa, Veronica Demarco Lima, Renata Nascimento, Alexandra Maria Vieira Monteiro, Egberto Gaspar de Moura, Celly Cristina Alves do Nascimento Saba |
|
|
Keywords: Computed tomography, Abdominal fat tissue, Malnutrition, Soy oil, Canola oil, Rats |
|
|
Abstract:
INTRODUCTION The intra-abdominal fat tissue (bodily fat surrounding visceral organs) is associated with adverse effects to the health, independently from the amount of bodily fat(1,2). The high accumulation of fat tissue in the intra-abdominal cavity is associated with hypertension, as reported by the African American Ethnicity Study(3), and with the risk for arteriosclerosis in non-obese Japanese individuals, i.e., with a normal body mass index (BMI)(4). Obesity is a condition recognized as a public health issue affecting the adult population in the United States and worldwide, increasing the risk for chronic diseases and decrease in the life expectancy(5,6). Although obesity has an influence on insulin resistance and diabetes mellitus, the rate of these metabolic alterations is high in the urban population in India where the individuals BMI is lower, and the waist/hip ratio is higher than in the European population(1), demonstrating that the fat tissue distribution within the abdominal region is as significant as is the BMI for the risk for metabolic disorders(4,7). The presence of intra-abdominal fat tissue also has been observed in non-obese children and adolescents(2). Computed tomography (CT) studies have demonstrated that, independently from the abdominal, subcutaneous fat tissue deposition, the 1 cm2-annual increase in the area of visceral fat tissue is associated with an increase of approximately 5% in the blood levels of insulin at fasting in North American children(8). The availability of in vivo imaging techniques has brought significant advantages for the study of the intra-abdominal fat tissue physiology(9). Abdominal CT is considered as the gold standard in the evaluation of the amount of subcutaneous and visceral fat tissue in this region(1,10). However, up to the present moment, there is no scientific report on CT in the evaluation of abdominal fat tissue following treatment for malnutrition in children. More than 50% of the mortality in children between 0 and 4 years of age is associated with malnutrition(11). In the treatment for this condition, the availability of a highly dense diet is frequently achieved with an increase in the dietary lipid content, especially with the addition of vegetal oils(12,13). The present study was aimed at describing, by means of CT, the repercussion of experimental post-weaning dietary supplementation with soy or canola oil on the abdominal fat distribution in previously undernourished rats.
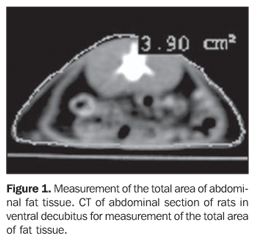
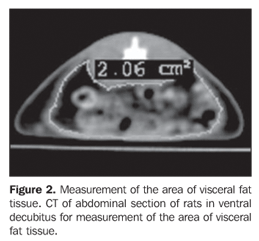
MATERIALS AND METHODS The sample of the present study included female Wistar rats. At three months of age, the animals were mated and, after that, were kept in cages with free access to water and food up to the litter birth. On the litter birth date (day 0), the dams were divided into two groups: a) control (C; n = 2), fed with a commercial standard diet, ad libitum; b) group submitted to 50% food restriction (FR; n = 6), in comparison with the previous day's diet given to the dams in group C. On the litter birth date, the litters were reduced to six male rat pups/litter to improve the lactation performance(14), until the end of the lactation period (day 21). The utilization of male rat pups was aimed at avoiding the influence of female steroids cycle that may represent an additional variable. After the weaning, the undernourished animals received an AIN-93G-type purified, hyperlipidic diet(15), supplemented with 19 g of soy oil (FR-soy 19%; n = 12) or canola oil (FR-canola 19%; n = 12) and 49 g of saccharose / 100 g chow, while the animals in the control group received the same purified diet supplemented with 7 g of soy oil and 60 g of saccharose / 100 g chow (C-soy 7%; n = 12). The three diets included 20 g of casein as a major protein source / /100 g chow. At 60 days of age, the rats were submitted to analysis of the fat tissue distribution by CT, with a GE HiSpeed helical model of the Unidade Docente-Assistencial de Radiologia, Centro Universitário de Controle do Câncer (CUCC/HUPE/UERJ), based on the protocol of helical acquisition, with axial 3 mm-thick slices, and 1.5 mm collimation. Measurements of intra-abdominal and intraperitoneal fat tissue thickness were performed, according to Yoshizumi et al.(10). For the purpose of this procedure, the rats received anesthesia with sodic pentobarbital (Thiopentax®, Cristália) and positioned in ventral decubitus on the apparatus table, according to the technique validated for rats(16). The images analysis was performed with the software DicomWorks v1.3.5(17), by means of automatic calculation of the area in cm2 of intra-abdominal and intraperitoneal fat tissue, always in the same axial plane. The abdominal circumference (total area including visceral and subcutaneous fat tissues) and the intraperitoneal (visceral) area were delineated with the cursor aiming at obtaining the area of intra-abdominal and intraperitoneal fat tissue(18) (Figures 1 and 2).
Immediately after CT examination, with the rats still under the effect anesthesia, measurements of their bodily length (cm) and mass (g) were performed before their sacrifice by decapitation. The rat's visceral fat was excised and weighted (absolute mass expressed in grams). Later, the absolute mass was adjusted in relation to the bodily mass (relative mass expressed in percentage of the absolute mass, divided by the bodily mass). The present study was developed in compliance with the ethical principles for animal experimentations adopted by the Colégio Brasileiro de Experimentação Animal (Cobea) (Brazilian College of Animal Experimentation), and approved by the ethical committee for care and use of experimental animals of Instituto de Biologia Roberto Alcântara Gomes (Ibrag) - Universidade do Estado do Rio de Janeiro (UERJ). The data collected were analyzed with the variance analysis method (Anova), and a post-test Newman-Keuls pairwise comparison. All the results were expressed as mean ± mean standard error (MSE), considering the significance level p < 0.05.
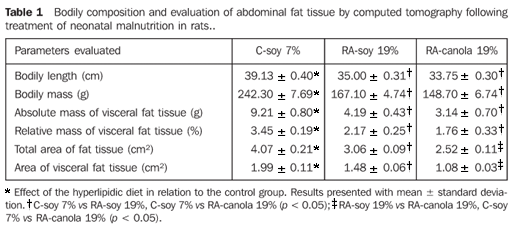
RESULTS The animals' evaluation following the experimental treatment demonstrated that both groups (FR-soy 19% and FR-canola 19%) achieved similar length and bodily mass recovery. As regards the absolute and relative mass of visceral fat tissue, none of the groups presented a significant difference. As it may be observed on Table 1, all of the evaluations performed in groups FR-soy 19% and FR-canola 19% demonstrated significantly lower results in comparison with group C-soy 7%.
The analysis of CT images demonstrates that total and visceral areas of group FR-canola 19% were respectively 17% and 27% smaller as compared with group FR-soy 19% (Table 1).
DISCUSSION During the experimental treatment for malnutrition, the reconstitution of the bodily fat tissue is influenced by the type of vegetal oil present in the hyperlipidic diet, the development of adipocytes in the different bodily compartments depend on the lipid source ingested(27,28). However, no previous study has demonstrated by means of CT that, despite the similar amount of visceral fat tissue mass, the lipid sources utilized - soy e canola oil - resulted in a difference in the fat tissue distribution within the abdominal cavity. Additional researches are necessary to understand how the abdominal fat tissue distribution during the early physical development will affect the individual's adult life. With the undernourishment during the lactation, the animals, after the weaning, have not developed hyperfagia and, consequently, have not presented nutritional recovery during the experimental treatment with the hyperlipidic diet. These results may supplement our previous results demonstrating that, during neonatal undernourishment, there is a decrease in the total volume of mother's milk, affecting the process of nutrients selection of the litter after weaning(24-26). However, other studies involving rats have shown that, in case of malnutrition during the gestation or after the lactation period, the animal can recover their bodily mass(19-23). Several techniques for evaluating the abdominal fat tissue have been developed. Anthropometric measurements, like evaluation of cutaneous folds, circumference of several bodily segments and the waist/hip ratio constitute simple and useful indicators for evaluation of abdominal fat tissue deposition. However, not always these indices are accurate. The CT, as a method for measuring the fat tissue distribution, allows an appropriate differentiation between subcutaneous and visceral fat tissues, which is unfeasible with conventional anthropometric techniques(7). This technique has been adopted for evaluation of the visceral fat tissue in adults(1) and in obese children(2).
CONCLUSION The visceral fat tissue evaluation by CT is more accurate than anatomic measurements, allowing the distinction of the fat tissue distribution within the abdominal cavity, as a function of the type of vegetal oil utilized - soy or canola - following experimental treatment of undernourished rats during lactation Acknowledgements Dr. Rodolfo Acatauassu Nunes, Coordinator for the CUCC Implementation Commision; Professor Dr. Gilson Telles Boa-Ventura, Coordinator for the Experimental Nutrition Laboratory at Faculdade de Nutrição - Universidade Federal Fluminense; Professor Dr. Eliete Bouskela, Head of the Ibrag Laboratory of Microcirculation - Universidade do Estado do Rio de Janeiro.
REFERENCES 1. Anjana M, Sandeep S, Deepa R, Vimaleswaran KS, Farooq S, Mohan V. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care 2004;27: 2948–2953. [ ] 2. Goran MI. Measurement issues related to studies of childhood obesity: assessment of body composition, body fat distribution, physical activity, and food intake. Pediatrics 1998;101:505–518. [ ] 3. Ding J, Visser M, Kritchevsky SB, et al. The association of regional fat depots with hypertension in older persons of white and African American ethnicity. Am J Hypertens 2004;17:971–976. [ ] 4. Miyawaki T, Abe M, Yahata K, Kajiyama N, Katsuma H, Saito N. Contribution of visceral fat accumulation to the risk factors for atherosclerosis in non-obese Japanese. Intern Med 2004;43: 1138–1144. [ ] 5. Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res 2002;10 Suppl 2:97–104. [ ] 6. James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res 2001;9 (Suppl 4):S228–S233. [ ] 7. Yucel A, Degirmenci B, Acar M, Albayrak R, Haktanir A. The effect of fasting month of Ramadan on the abdominal fat distribution: assessment by computed tomography. Tohoku J Exp Med 2004;204:179–187. [ ] 8. Huang TTK, Johnson MS, Gower BA, Goran MI. Effect of changes in fat distribution on the rates of change of insulin response in children. Obes Res 2002;10:978–984. [ ] 9. Goran MI, Kaskoun M, Shuman WP. Intra-abdominal adipose tissue in young children. Int J Obes 1995;19:279–283. [ ] 10. Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology 1999;211:283–286. [ ] 11. Ashworth A. Treatment of severe malnutrition. J Pediatr Gastroenterol Nutr 2001;32:526–518. [ ] 12. Bhan MK, Bhandari N, Bahl R. Management of the severely malnourished child: perspective from developing countries. BMJ 2003;326:146–151. [ ] 13. Lacerda EMA, Faria IG. Desnutrição energético-protéica na infância. In: Accioly E, Saunders C, Lacerda EMA. Nutrição em obstetrícia e pediatria. 1ª ed. Rio de Janeiro, RJ: Cultura Médica, 2002;444. [ ] 14. Fishbeck KL, Rasmussen K. Effect of repeated cycles on maternal nutritional status, lactational performance and litter growth in ad libitum-fed and chronically food-restricted rats. J Nutr 1987; 117:1967–1975. [ ] 15. Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 1997; 127:838–841. [ ] 16. Ross R, Léger L, Guardo R, Guise JD, Pike BG. Adipose tissue volume measured by magnetic resonance imaging and computerized tomography in rats. J Appl Physiol 1991;70:2164–2172. [ ] 17. Pellegrinetti B, Magna LA. Sobre uma metodologia de apresentação de imagem médica. Radiol Bras 2004;37:211–213. [ ] 18. Spina LDC, Soares DV, Conceição FL, et al. Avaliação do metabolismo glicídico e da gordura visceral em adultos deficientes de hormônio de crescimento. Arq Bras Endocrinol Metab 2002;46: 536–543. [ ] 19. Dullo AG, Mensi N, Seydoux J, Girardier L. Differential effects of high-fat diets varying in fatty acid composition o the efficiency of lean and fat tissue deposition during weight recovery after low food intake. Metabolism 1995;44:273–279. [ ] 20. Shillabeer G, Lau DCW. Regulation of new fat cell formation in rats: the role of dietary fats. J Lipid Res 1994;35:592–600. [ ] 21. Vicente LL, Moura EG, Lisboa PC, et al. Malnutrition during lactation in rats is associated with higher expression of leptin receptor in the pituitary of adult offspring. Nutrition 2004;20:924–928. [ ] 22. Passos MCF, Ramos CF, Dutra SCP, Mouço T, Moura EG. Long-term effects of malnutrition during lactation on the thyroid function of offspring. Horm Metab Res 2002;34:40–43. [ ] 23. Passos MCF, Ramos CF, Teixeira CV, Moura EG. Comportamento alimentar de ratos adultos submetidos à restrição protéica cujas mães sofreram desnutrição durante a lactação. Rev Nutr Campinas 2001;14:7–11. [ ] 24. Langley-Evans SC, Bellinger L, McMullen S. Animal models of programming: early life influences on appetite and feeding behaviour. Matern Child Nutr 2005;1:142–148. [ ] 25. Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr 2004;92:513–520. [ ] 26. Hales CN, Ozanne SE. The dangerous road of catch-up growth. J Physiol 2003;547:5–10. [ ] 27. Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proceedings of the Nutrition Society 2000;59:359–371. [ ] 28. Harris PM. Changes in adipose tissue of the rat due to early undernutrition followed by rehabilitation. Br J Nutr 1980;43:15–26. [ ]
Received December 19, 2006. Accepted after revision February 13, 2007.
* Study developed in the Department of Physiological Sciences of Instituto de Biologia Roberto Alcântara Gomes (Ibrag), Centro Universitário de Controle do Câncer (CUCC), Hospital Universitário Pedro Ernesto da Universidade do Estado do Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554