Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 5 - Sep. / Oct. of 2007
Vol. 40 nº 5 - Sep. / Oct. of 2007
|
ORIGINAL ARTICLE
|
|
Lipoid pneumonia in adults: findings on high-resolution computed tomography |
|
|
Autho(rs): Edson Marchiori, Gláucia Zanetti, Dante L. Escuissato, Arthur Soares Souza Jr., César Araújo Neto, Luiz Felipe Nobre, Klaus L. Irion, Rosana Rodrigues, Alexandre Dias Mançano, Domenico Capone, Suzane Mansur Fialho, Carolina Althoff Souza |
|
|
Keywords: Exogenous lipoid pneumonia, High-resolution computed tomography, HRCT, Aspiration pneumonia, Lungs |
|
|
Abstract:
INTRODUCTION Several pulmonary complications may be caused by aspiration of different substances into the airways and lungs. Exogenous lipoid pneumonia is a rare condition resulting from inhalation or aspiration of animal, vegetal or mineral oil(1). The most frequent presentation of lipoid pneumonia is the one caused by aspiration of mineral oil, often orally utilized to treat chronic constipation(2). The clinical presentation is non-specific and is associated with the patient's age, the aspirated volume, and if the aspiration was acute or chronic(3). Exogenous lipoid pneumonia diagnosis is based on the history of exposure to oil, on compatible chest radiograph and/or computed tomography (CT), and on the presence of macrophages with fat droplets in sputum or in the bronchoalveolar lavage. Transbronchial or open biopsy may be necessary in case the diagnosis remains undefined(4). High resolution computed tomography (HRCT) plays an essential role in the investigation. The present study was aimed at presenting the HRCT findings observed in adult patients with exogenous lipoid pneumonia caused by mineral oil aspiration.
MATERIALS AND METHODS CT images of eight adult patients with confirmed diagnosis of exogenous lipoid pneumonia were submitted to retrospective, descriptive, observational analysis. The CT images were randomly selected from the nosologic archives of eight medical institutions in five different Brazilian states in the period between March/1994 and June/2006. Four patients were women and four men, with ages ranging between 46 and 88 years, mean 69.4 years. All of them presented with cough, four associated with dyspnea. As regards predisposing factors, two patients had megaesophagus, one, gastroesophagel reflux disease, one Parkinson's disease, and another, cerebral palsy. A sixth patient complained of frequent choking. In four patients, the diagnosis was confirmed by bronchoalveolar lavage; one patient was submitted to pulmonary biopsy; and, in three patients the diagnosis was reached by means of the association of the clinical picture with the finding of fat densities at HRCT. At the moment of examination, no patient presented clinical or laboratory evidence of associated infection. All of them utilized oral mineral oil as a laxative agent. Considering the multiple institutions involved, the studies were performed in different tomographs, all of them with the high resolution technique, with slices from the apex through the pulmonary bases. Images were acquired with thin slices (1 mm or 2 mm-thick), with the patients in dorsal decubitus, during inspiration, with a high spatial resolution filter (bone filter) for images reconstruction, 10 mm increment, without intravenous infusion of iodinated contrast media. The images were acquired and reconstructed on a 512 × 512 matrix and photographed for evaluation of pulmonary fields with windows opening ranging between 1200 UH and 2000 UH, level between 300 UH and 700 UH. For evaluation of the mediastinum, the windows variation was between 350 UH and 500 UH, and center variation, between 10 UH and 50 UH. The images were blindly reviewed by two independent observers with consensus agreement.
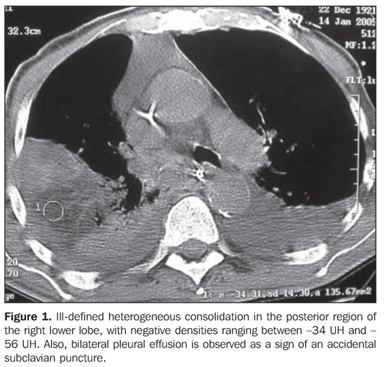
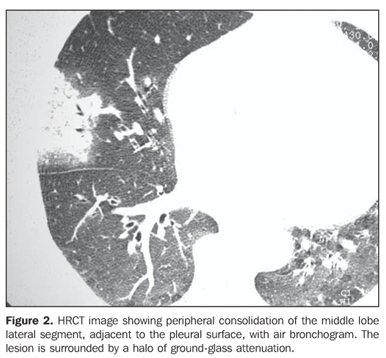
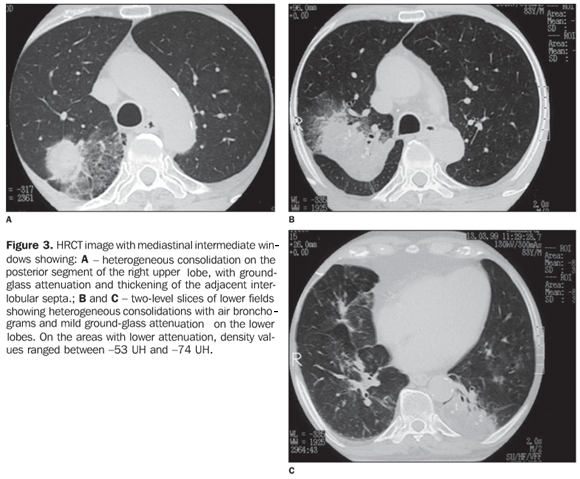
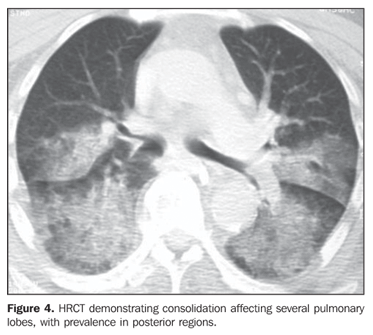
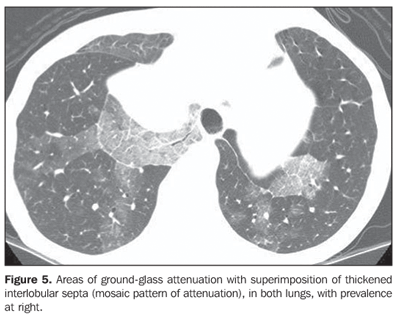
RESULTS The most frequent tomographic findings were consolidations and ground-glass attenuation, associated or not with thickening of interlobular septa (mosaic pattern attenuation). Consolidation with air bronchogram and fat densities interposition was observed in six patients; two of these cases with single findings, on in the right lower lobe (Figure 1), and the other in the middle lobe (Figure 2). In the other patients, multiple consolidations were found (Figures 3 and 4). In four cases, consolidations were surrounded by areas of ground-glass attenuation. All of the consolidations presented areas with fat density (-34 UH to -74 UH) inside.
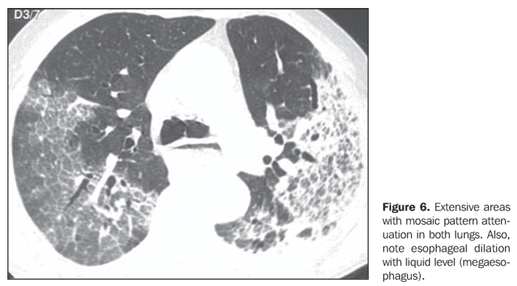
Two patients presented areas of ground-glass attenuation associated with interlobular septa thickening, characterizing the mosaic pattern of attenuation (Figures 5 and 6). In three cases, interlobular septa thickening was observed, two of them with mosaic pattern attenuation, and one in the periphery of one of the consolidation areas. Disease-related pleural or lymph node involvement was absent in all of the patients. The lesions were bilateral in six patients, predominating in posterior regions, and unilateral in two patients.
DISCUSSION Exogenous lipoid pneumonia is an uncommon condition, resulting from oil inhalation or aspiration into the lungs(1). The chronic presentation of the disease results from repeated oil inhalation or aspiration for a long period of time, and the acute presentation is secondary to accidental and massive aspiration of lipid materials, like that traditionally described in fire swallowers (performers who pretend to swallow or spit fire)(5,6). The oils may be of animal, vegetal or mineral nature(2). The majority of cases occur by mineral oil inhalation or aspiration(2). In adults, the most frequent cause of exogenous lipoid pneumonia is the utilization of mineral oil to treat intestinal constipation, followed by the use of oily nose drops to treat chronic rhinitis(7). Annually, in the United States, between two and three million persons receive cathartic and laxative agents prescribed by doctors. Additionally, self-medication should be taken into consideration, since mineral oil is an over-the-counter drug(3). The amount of mineral oil ingested may be surprising. In Brazil, mineral oil is freely available in public health units, greatly facilitating its use(8). Gastrointestinal diseases, esophagogastric fistula, psychiatric diseases, neurological diseases that change the deglutition or cough reflex, and loss of consciousness constitute predisposing factors for aspiration of oropharyngeal or gastric contents. The supernatant oily material remains within the stomach, and, when aspirated, preferentially enters the airways(2,9,10). Usually, mineral oil aspiration is imperceptible, due the absence of airways-protective reflexes like cough and glottic closing(11). In the case of oily nose drops, they may easily and silently reach the bronchial tree of a sleeping individual without triggering cough reflex(7), besides being hardly expelled because they impair the normal mucociliary transport(12). Macrophages phagocyte the oily material and gradually increase in number, filling alveolar spaces. Then, they are incorporated into the alveolar walls, and, through the lymphatic channels, reach the interlobular septa causing its thickening(11,13,14). The sometimes extensive fibrotic proliferation secondary to repeated aspiration(11,14), may be associated with a considerable loss of pulmonary volume(15). Fibrosis may develop and the oil may coalesce, forming large fat drops involved by a fibrotic tissue and giant cells, creating a tumor-like mass called paraffinoma(4). Clinical presentation is non-specific, varying with the age, amount and quality of the oil aspirated, and if the aspiration was chronic or acute(3,16,17). As regards symptoms, from asymptomatic patients to severe, life-threatening cases can be observed(17). In elderly, these pneumonias usually develop progressively, becoming chronic, with no clinical repercussion, and may be found at necropsy(18). Most of times, clinical and radiographic findings are dissociated in cases of lipoid pneumonia. While the patients are asymptomatic there are plenty of incidental radiographic findings on routine radiographies(1,8). The overall clinical findings may mimick bacterial pneumonia, with a sudden episode of fever and cough(19). Most frequently, lipoid pneumonia presents with symptoms of, sometimes productive, chronic cough, and dyspnea. Chest pain, hemoptysis, weight loss, and intermittent fever are less frequent findings(2). Although a history of oil ingestion or inhalation constitutes an extremely relevant data, rarely it is spontaneously informed by the patient, making the diagnosis more difficult(13,16). Many times, this information is retrospectively obtained, after directed anamnesis. Considering that lipoid pneumonia clinical and radiological findings are non-specific, it may mimick several other diseases(3), especially lung cancer(20). The exogenous lipoid pneumonia diagnosis is based on the history of exposure to oil, compatible radiological studies, and the presence of macrophages with fat droplets in the sputum or bronchoalveolar lavage. If the diagnosis remains undefined, transbronchial or open biopsy may be required(4). In conventional radiology, findings of lipoid pneumonia are poorly specific(13), and the disease alterations appear with variable patterns and distribution(15). HRCT is the best imaging method for lipoid pneumonia diagnosis(20). CT may demonstrate alveolar consolidations, ground-glass attenuation, interstitial abnormalities, interlobular septa thickening, intralobular interstitial thickening, and nodular lesions (small, ill-defined centrolobular nodules(10,11). Mosaic pattern of attenuation, corresponding to interlobular septa thickening superimposing ground glass attenuation is frequently observed(1,21,22). The most typical sign of this disease is the presence of pulmonary consolidations with fat attenuation(1,3,18,22), i.e., negative attenuation values(7), particularly when associated with a history of exposure to oil, that contributes for the definition of the diagnosis. Negative densities ranging between -150 UH and -30 HU are highly suggestive of intrapulmonary fat(3,23). These measures may not be precise in the presence of pulmonary fibrosis and/or inflammatory exudate involving the oily material, which increases the attenuation values(11,18).
REFERENCES 1.Laurent F, Philippe JC, Vergier B, et al. Exogenous lipoid pneumonia: HRCT, MR, and pathologic findings. Eur Radiol 1999;9:1190–1196. [ ] 2.Spickard A, Hirschmann JV. Exogenous lipoid pneumonia. Arch Intern Med 1994;154:686–692. [ ] 3.Bandla HPR, Davis SH, Hopkins NE. Lipoid pneumonia: a silent complication of mineral oil aspiration. Pediatrics 1999;103:E19. [ ] 4.Adkins D, Bensadoun ES. An 85-year old man with a lung mass. Chest 2004;125:1121–1123. [ ] 5.Bernabeu Mora R, Méndez Martínez P, Abellán Martínez MC, Polo García LA, Lorenzo Cruz M, Sánchez Gascón F. Neumonía lipoidea aguda debida a la aspiración accidental de vaselina utilizada en un sondaje nasogástrico. Arch Bronconeumol 2000;36:485–487. [ ] 6.Gentina T, Tillie-Leblond I, Birolleau S, et al. Fire-eaters lung: seventeen cases and a review of the literature. Medicine 2001;80:291–297. [ ] 7.Franquet T, Giménez A, Rosón N, Torrubia S, Sabaté JM, Pérez C. Aspiration diseases: findings, pitfalls, and differential diagnosis. RadioGraphics 2000;20:673–685. [ ] 8.Ranzani MF, Miranda NS, Junior UF, Ribeiro SM, Machado JM. Pneumonia lipoídica associada à forma digestiva da doença de Chagas. J Bras Pneumol 2004;30:492–495. [ ] 9.Pérez Payá A, Martínez Serrano C, López Andreu JA, Cortell Aznar I, Roqués Serradilla JM. Neumonía de evolución tórpida. An Pediatr (Barc) 2003;58:619–620. [ ] 10.Baron SR, Haramati LD, Riviera VT. Radiological and clinical findings in acute and chronic exogenous lipoid pneumonia. J Thorac Imaging 2003;18:217–224. [ ] 11.Farias J, Martins EML, Pozes AS, Fialho SM, Marchiori E. Pneumonia lipídica – aspectos na tomografia computadorizada: relato de caso. Radiol Bras 2004;37:57–60. [ ] 12.Gondouin A, Manzoni PH, Ranfaing E, et al. Exogenous lipid pneumonia: a retrospective multicentre study of 44 cases in France. Eur Respir J 1996;9:1463–1469. [ ] 13.Lee KS, Müller NL, Hale V, Newell JD Jr, Lynch DA, Im JG. Lipoid pneumonia: CT findings. J Comput Assist Tomogr 1995;19:48–51. [ ] 14.Pinkerton H. The reaction to oils and fats in the lung. Arch Pathol 1928;5:380–401. [ ] 15.Lee JY, Lee KS, Kim TS, et al. Squalene-induced extrinsic lipoid pneumonia: serial radiologic findings in nine patients. J Comput Assist Tomogr 1999;23:730–735. [ ] 16.Midula F, Strappini PM, Ascoli V, et al. Bronchoalveolar lavage cell analysis in a child with chronic lipid pneumonia. Eur Respir J 1998;11:239–242. [ ] 17.Soloaga ED, Beltramo MN, Veltri MA, Ubaldini JE, Chertcoff FJ. Insuficiencia respiratoria aguda por neumonia lipoidea. Medicina (Buenos Aires) 2000;60:602–604. [ ] 18.Malheiros NR, Marchiori E, Praxedes MC, Machado DM, Morandi JLJB, Teixeira GHMC. Pneumonia por aspiração de óleo mineral: relato de um caso. Radiol Bras 1995;28:213–216. [ ] 19.Abad Fernández A, Miguel Díez J, López Vime R, Gómez Santos D, Nájera Botello L, Jara Chinarro B. Neumonía lipoidea en relación con exposición laboral a pinturas. Arch Bronconeumol 2003;39:133–135. [ ] 20.Bréchot JM, Buy JN, Laaban JP, Rochemaure J. Computed tomography and magnetic resonance findings in lipoid pneumonia. Thorax 1991;46: 738–739. [ ] 21.Rossi SE, Erasmus JJ, Volpacchio M, Franquet T, Castiglioni T, MacAdams HP. "Crazy-paving" pattern at thin-section CT of the lungs: radiologic-pathologic overview. RadioGraphics 2003;23: 1509–1519. [ ] 22.Franquet T, Giménez A, Bordes R, Rodriguez-Arias JM, Castella J. The crazy-paving pattern in exogenous lipoid pneumonia: CT-pathologic correlation. AJR Am J Roentgenol 1998;170:315–317. [ ] 23.Wheeler PS, Stitik FP, Hutchins GM, Klinefelter HF, Siegelman SS. Diagnosis of lipoid pneumonia by computed tomography. JAMA 1981;245: 65–66. Radiol Bras 2007;40(5):315–319 [ ]
Received December 2, 2006. Accepted after revision January 8, 2007.
* Study developed in the Service of Radiodiagnosis at Hospital Universitário Clementino Fraga Filho (HUCFF) da Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554