Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 3 - May / June of 2007
Vol. 40 nº 3 - May / June of 2007
|
ORIGINAL ARTICLE
|
|
Gastrointestinal stromal tumor: clinical, radiologic and pathologic features |
|
|
Autho(rs): Leonardo Lopes de Macedo, Lucas Rios Torres, Rafael Artigas Faucz, Olger de Souza Tornin, Fábio Mota Gonzalez, Igor Motta de Aquino, Carlos Alberto Marcovechio Fonseca, Alexandre Pescioto, Ricardo Pires de Souza |
|
|
Keywords: Gastrointestinal stromal tumor, Gastrointestinal neoplasms, Gastrointestinal diseases, Sarcoma |
|
|
Abstract:
IMD, Resident in Radiology at the Service of Radiology and Diagnostic Imaging, Fellow Master degree in Sciences of Health, Post-graduation at Hospital Heliópolis, São Paulo, SP, Brazil
INTRODUCTION Gastrointestinal stromal tumors (GIST) are the most frequent mesenchymal neoplasms of the gastrointestinal tract, characterized by the expression of the C-KIT protein (CD117), a membrane receptor with a tyrosine kynase component(1–3). Although they may occur in any site of the gastrointestinal tract, they correspond to only 1% of tumors in these organs(2). These tumors affect subjects above 50 years of age, and rarely are found before the age of 40 years(4). Symptoms are non-specific, and computed tomography (CT) is the method of choice for the diagnosis of this lesion(5). Previously, GISTs were classified into a group of smooth muscle tumors including leiomyomas, leiomyosarcomas, and leiomyoblastomas(2). With the introduction of immunohistochemical staining techniques and the breakthrough of markers such as the C-KIT, currently these tumors are recognized as a distinct, new class of tumors, which is extremely important, considering the differences in their prognosis and treatment(6). GISTs presentations may range from small, asymptomatic, incidentally detected lesions to masses large enough to cause symptoms, including multiple metastases(2). Metastases, usually, affect the liver and the peritoneum, but rarely lymph nodes(1,5,7). In case of localized tumors, surgical resection is the therapy of choice(5). In patients with inoperable or metastatic disease, immediate imatinib therapy (STI571 — a tyrosine kynase inhibitor) is indicated(5,7,8). Considering that this is a recently described disease, we have tried to demonstrate the relevance of imaging studies in the detection of these tumors, besides evaluating the role of these methods for aiding in the differential anatomopathological diagnosis.
MATERIALS AND METHODS The present study was retrospectively performed, utilizing the non-experimental (observational) model. Data from 16 patients operated for GIST in our institution in the period between December/2000 and March/2006 were evaluated. Only lesions with histopathological and immuno-histochemical (C-KIT-positive) patterns compatible with GIST were included. All the CKIT-negative patients were excluded. The variables analyzed were the following: patients' sex and age, signs and symptoms at the initial presentation, primary site and size of the tumor, radiological findings, anatomopathological features of the lesion, presence of metastasis at diagnosis, and incidence of metastasis or tumor recurrence in the follow-up of the patients. Imaging studies (12 CT and two esophagogastroduodenal – EGD series) of 12 patients in the sample of 16 were evaluated by two radiologists (specialist title by Colégio Brasileiro de Radiologia e Diagnóstico por Imagem) of our institution. From the other four patients whose CT studies were not available, we could only to recover the CT reports. The radiological signs evaluated were: site and size of the lesion, contrast-enhancement, margins, contours, central hypodensity, calcification and presence of metastases.
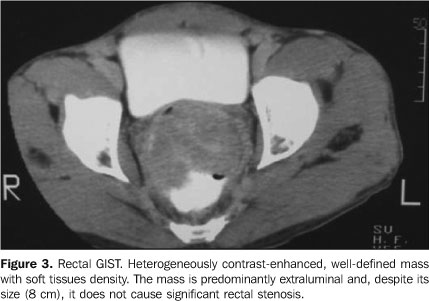
RESULTS The study population included nine men and seven women. Mean age among men was 49 years (ranging between 25 and 66 years), and among women was 69 years (ranging between 63 and 75 years). The group mean age was 58 years. Initially, the main complaints of patients were: body weight loss (n = 8), followed by abdominal pain (n = 7), nausea (n = 5), emesis (n = 3), upper digestive hemorrhage (n = 1), hematochezia (n = 1) and melena (n = 1). Two patients were admitted into the hospital with acute obstructive abdomen, and one with intestinal subocclusion. In the present study, primary tumors sites of origin were: stomach (n = 5), rectum (n = 4), small bowel (n = 3), mesentery (n = 3) and sigmoid (n = 1). The tumors size ranged between 2 cm and 20 cm (mean = 9 cm), with stomach tumors presenting mean 3 cm and the mesenteric ones (Figure 1), mean 15 cm. At CT all of the tumors presented heterogeneous contrast-enhancement. The gastric neoplasms presented circumscribed margins and slightly lobulated contour, with only one tumor (the largest, with 6 cm) with a central hypodense area. The mesenteric tumors, as well as the small bowel tumors (Figure 2), produced a mass effect causing compression of adjacent structures. They were larger, with lobulated margins, and only one of them did not present a central hypodense area. A mural mass causing the mucosa to bulge, with mildly lobulated margins was the main presentation of rectal tumors (Figure 3). Calcification was found in two mesenteric tumors.
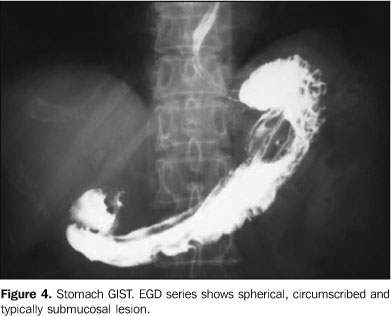
In both cases evaluated by EGD series, a circumscribed, typically submucosal lesion without signs of ulceration was found (Figure 4).
Histopathologically, 13 patients presented with spindle cells tumors (Figure 5A), and other three with epithelioid cells (one in the small bowel, and two in the rectum). The immunohistochemical analysis was essential for diagnosis confirmation, and in all of the cases presented C-KIT positivity (Figure 5B). Four patients present with liver metastasis at the moment of the diagnosis and other five patients presented tumor recurrence, four of them in the peritoneum, and one in the liver, within two months to eight years and eight months (mean period = two years and eight months). Liver metastases were hypoattenuating as compared with the well-defined, adjacent normal parenchyma.
DISCUSSION GISTs are the most frequent mesenchymal neoplasms occurring at any site of the gastrointestinal tract(1,3). Approximately 40%–70% of GISTs affect the stomach, accounting for 2.5% of gastric tumors, 20%–40% affect the small bowel, and the remainders occur in other sites such as esophagus, colon, rectum, mesentery and omentum(9,10). These tumors affect subjects above 50 years of age, and rarely are found before the age of 40 years, with a slightly higher male prevalence(4,7). In the present casuistic, the mean age at the moment of the diagnosis amongst men was markedly lower than amongst women, raising the hypothesis that GISTs affect men at an earlier age. This data is not reported in the literature. Clinical symptoms are non-specific and are basically associated with the site and size of the lesion. Abdominal pain, distension, gastrointestinal bleeding, anemia, body weight loss and palpable mass are some of possible signs of the disease(4,7). These tumors may achieve large dimensions, with size usually ranging between 3 cm and 10 cm(1), and because of a predominantly extraluminal growth, they rarely cause obstructive symptoms(1,4,11). In the present study, stomach tumors presented considerably smaller at diagnosis, as compared with the mesenteric tumors, which corroborates the literature(12,13). Histologically, GISTs are classified according to the predominant cellular type, as follows: spindle cells (70%), epithelioid cells (20%), and mixed (10%)(6). Immuno-histochemical evaluation may detect the C-KIT (CD117), a tyrosine kynase receptor, the most important GIST marker(6,14). The majority of lesions also present CD34 positivity. Other possible markers include vimentine, actin, S-100 protein and (rarely) desmin(1,4,6,9). These markers are extremely useful in the differentiation of these tumors from others of similar origin, such as leiomyomas, leiomyoblastomas, leiomyosarcomas, and schwannomas(3). Some tumors such as leiomyosarcomas may show radiological and histological presentations very similar to GISTs, however C-KIT is GIST-specific(15). GIST may be benign or malignant, and major negative prognostic factors include distal intestinal location, tumor size, high mitotic activity, and presence of metastasis(6,10). There is no correlation between degree of necrosis, hemorrhage or pattern of contrast-enhancement on CT indicating a higher or lower malignant potential(4). Notwithstanding some studies demonstrate that less than 50% of primary, localized tumors do not recur in a five-year-period(10), it is known that in cases of tumor recurrence in the liver or peritoneum (the two most frequent sites of metastasis) the prognosis is poor(6). In the present study, nine patients (56% of cases) presented with metastasis at diagnosis, or tumor recurrence in a mean period of two years and eight months, which demonstrates a high propensity to malignancy. Considering that this is a recently described disease, studies reporting a long lasting follow-up of a considerable number of patients are still to be published. Currently, these tumors are considered as potentially malignant and, therefore, all the patients affected by this disease should be carefully treated and followed-up(5,6,10). Amongst the currently available imaging methods, CT remains as the method of choice for evaluation of abdominal masses or biopsy-confirmed GISTs, especially if the wide availability of the method is considered(5). Generally, these tumors present as a well circumscribed mass, frequently originating from the stomach or small bowel, with heterogeneous contrast-enhancement(7,11). Small foci of calcifications, frequently related to malignant lesions may be observed(12). Areas of central attenuation may correspond to cystic degeneration, hemorrhage or tumor necrosis(4,9,13), which includes this neoplasm in the differential diagnosis of cystic or necrotic lesions related to the stomach or adjacent structures(11). Mucosal alteration may be found in up to 50% of gastric tumors(1) and aneurismatic dilatation of small bowel loops, previously related to lymphoma, may be found in up to 33% of enteric GISTs(11). Most of times, mesenteric GISTs present well-defined margins, lobulated contour, large dimensions (10 cm to 27 cm) and areas of low central attenuation(13). In their most aggressive feature, these tumors may generate metastases, the liver and peritoneum being the most affected sites. More rarely, the tumor may spread to lymph nodes, bones and lungs. At CT, liver metastases present contrast-enhancement, because of their usually hypervascular nature(1,7). It is important to note that, during the CT portal phase, hepatic metastases may become imperceptible, which makes the performance of the arterial phase extremely important(5). The cystic pattern appearance after na adequate chemotherapy is typical and has already been described in the literature, and should not be erroneously interpreted as a disease progression or as new lesions(1,5,16). GISTs can be cured only by surgery(5). Considering the absence of a true capsule, the tumor must be block-resected with a free 2-3 cm margin as possible. Lymphadenectomy is unnecessary since these tumors rarely produce lymph nodes metastasis(2, 3,5). The follow-up of these patients must include CT every six months, considering the potentially malignant nature of the disease(5,10). In cases of inoperable or metastatic tumors, the therapy of choice is with imatinib (STI571), a tyrosine kynase inhibitor, and there is no indication for radiotherapy or chemotherapy. The drug administration should be initiated upon the diagnosis of metastatic or advanced disease, and maintained until the patient develops intolerance or progressive disease(3,5). Recent studies have demonstrated that more than 50% of patients with advanced disease are responsive to the medicamentous treatment(8,17,18). CT remains as the method of choice for evaluation of the patients´ response to the therapy, although positron emission tomography (PET) has shown high sensitivity for demonstrating na early response of the tumor(19). Progressive hypoattenuation of the mass, decrease in nodular and vascularization enhancement are parameters indicative of a good response of the tumor to the therapy(8). However, it should be highlighted that some tumors increase in size during the first six months of therapy, despite the significant clinical improvement and regression visualized by PET(5,19).
CONCLUSION GISTs, although relatively rare, are the most frequent mesenchymal neoplasms in the gastrointestinal tract. These tumors affect middle-aged adults and elders, and notwithstanding the patients present with non-specific symptoms, they should be considered in the differential diagnosis of solid of solid/cystic masses in the abdominal cavity. In the present study, the most frequent site of GISTs was the stomach. Gastric tumors presented reduced dimensions as compared with small bowel and mesenteric tumors. Central hypodensity was observed in 50% of cases and in larger tumors. Calcification was not a common finding. Occurrence of metastasis or tumor recurrence was observed in the majority of cases. The main finding at CT was heterogeneously contrast-enhanced circumscribed mass, with lobulated contour. The EGD series identified circumscribed and typically submucosal mass. These findings corroborate the literature(1,4,7). Two patients presented with acute obstructive abdomen, and one with intestinal subocclusion at the initial presentation. These are uncommon findings at the first presentation, however they should be considered in the differential diagnosis of abdominal lesions. Spindle cells pattern was the main histological tumor type, followed by the epithelioid cell type, also corroborated in the literature(4,6). As demonstrated in the present study, GISTs present a high tendency to malignancy. Therefore, early diagnosis, an appropriate therapy and careful follow-up are essential for the management of the disease. Finally, amongst the differential diagnoses, the radiologist's suspicion is essential to reduce the morbidity or even the mortality of patients with GIST.
REFERENCES 1. Sandrasegaran K, Rajesh A, Rydberg J, Rushing DA, Akisik FM, Henley JD. Gastrointestinal stromal tumors: clinical, radiologic, and pathologic features. AJR Am J Roentgenol 2005;184:803–811. [ ] 2. Sugár I, Forgács B, István G, Bognár G, Sápy Z, Ondrejka P. Gastrointestinal stromal tumors (GIST). Hepatogastroenterology 2005;52:409–413. [ ] 3. Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors. J Gastroenterol 2005;40:775–780. [ ] 4. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. RadioGraphics 2003;23:283–304. [ ] 5. Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16:566–578. [ ] 6. Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459–465. [ ] 7. Burkill GJC, Badran M, Al-Muderis O, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology 2003;226:527–532. [ ] 8. Dagher R, Cohen M, Williams G, et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res 2002;8:3034–3038. [ ] 9. Sharp RM, Ansel HF, Keel SB. Best cases of the AFIP: gastrointestinal stromal tumor. Armed Forces Institute of Pathology. RadioGraphics 2001;21:1557–1560. [ ] 10. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51–58. [ ] 11. Sandrasegaran K, Rajesh A, Rushing DA, Rydberg J, Akisik FM, Henley JD. Gastrointestinal stromal tumors: CT and MRI findings. Eur Radiol 2005;15:1407–1414. [ ] 12. Kim HC, Lee JM, Kim KW, et al. Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am J Roentgenol 2004;183:893–898. [ ] 13. Kim HC, Lee JM, Kim SH, et al. Primary gastrointestinal stromal tumors in the omentum and mesentery: CT findings and pathologic correlations. AJR Am J Roentgenol 2004;182:1463–1467. [ ] 14. Medeiros F, Corless CL, Duensing A, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 2004;28:889–894. [ ] 15. Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol 2000;24: 1339–1352. [ ] 16. Chen MY, Bechtold RE, Savage PD. Cystic changes in hepatic metastases from gastrointestinal stromal tumors (GISTs) treated with Gleevec (imatinib mesylate). AJR Am J Roentgenol 2002; 179:1059–1062. [ ] 17. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–480. [ ] 18. van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001;358:1421–1423. [ ] 19. Antoch G, Kanja J, Bauer S, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med 2004;45:357–365. [ ]
Received May 27, 2006.
* Study developed in the Service of Radiology and Diagnostic Imaging, Hospital Heliópolis, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554