Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 2 - Mar. / Apr. of 2007
Vol. 40 nº 2 - Mar. / Apr. of 2007
|
ORIGINAL ARTICLE
|
|
Ruthenium-106 brachytherapy for uveal melanomas - preliminary results: a single institutional experience |
|
|
Autho(rs): Rodrigo Souza Dias, Adelmo José Giordani, Clélia Maria Erwenne, Helena Regina Comodo Segreto, Luiz Fernando Teixeira, Roberto Araujo Segreto |
|
|
Keywords: Uveal melanoma, Ruthenium plaque therapy, Conservative therapy, Brachytherapy |
|
|
Abstract:
IRadiotherapy Unit of Department of Medicine – Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil
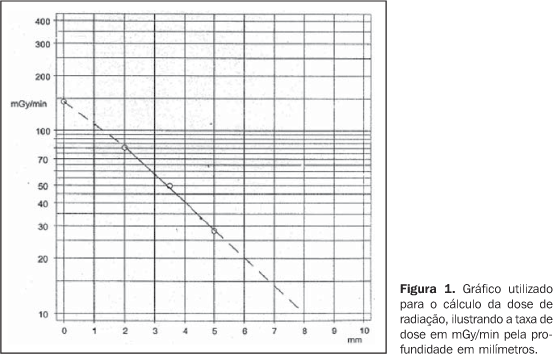
INTRODUCTION Although rare, uveal melanoma is the most common primaryintra-ocular malignancy in adults, with an approximate frequencyof six cases per million inhabitants per year in the UnitedStates(1,2), seven cases per millioninhabitants per year in the Western Europe(3).Estimates for Brazil are not available. Up to the eighties, the treatment for patients with uvealmelanoma was surgical, consisting in enucleation, still themainstay of therapeutical intervention for extensive lesions.However, other therapies aiming at preserving the vision and theocular globe have been proposed since1930(4). In 1930, Moore utilized radon seeds directly implanted intothe ocular tumor of a patient(4). In 1960,Stallard initiated the treatment for these tumors with cobalt-60episcleral plaques. Since then, other isotopes like iodine-125,iridium-192, gold-198, paladium-103 and ruthenium-106 startedbeing utilized(6–8). Randomized prospective studies like those developed by theCollaborative Ocular Melanoma Study (COMS) since 1986, showedthat the mortality rate of patients with melanomas between 2.5 mmand 10 mm in elevation was similar when compared with themortality rate for patients submitted to iodine-125 brachytherapyand to enucleation(6). Yet, in 1986, Lommatzschreported the utilization of ruthenium-106 beta-radiation fortreatment of uveal melanomas with results comparable to thosefrom other radioisotopes(7). So, ocularbrachytherapy started being used in patients with uveal melanomaswith up to 10 mm in elevation, allowing results similar to thosefrom more radical procedures like enucleation, while preservingthe visual acuity and the ocular globe. Amongst the radioisotopes most frequently utilized in ocularbrachytherapy, cobalt-60 is a gamma-radiation emitter with 1.25MeV energy and 5.2-year half-life, whose main disadvantages arethe higher radiation exposure of the medical team and higherradiation dose in adjacent organs, and iodine-125, agamma-radiation emitter with 0.028 MeV energy and a relativelyshort 59.6-day half-life. As regards ruthenium-106, this isotopepresents as main characteristic the beta-radiation emission,allowing higher dose concentration in the tumor, and lower dosein adjacent areas. Besides, the medical team involved in theprocedure is submitted to lower exposure to radiation, and organsin risk receive lower radiation doses, consequently optimizingthe radiological protection(3,8). Considering that ruthenium-106 is a beta-radiation emitter, it is best indicated in the treatment of small melanomas, with up to 6 mm in elevation and a long half-life, allowing longer periods of use and therefore lower cost compared with other isotopes. Ruthenium radiation dose calculation is performed with basis on tables provided by the manufacturer, and otherwise expensive computer planning systems are not necessary(9) (Figure 1).
Main complications associated with brachytherapy are:retinopathy, cataract, neovascular glaucoma and maculopathy whichmay occur in 9% to 27% of cases in a three-year period. Maininfluencing factors which seem to be related with suchcomplications are tumor elevation, TMN stadium, and the proximityof the tumor with the fovea and opticdisc(10). Most recently, transpupillary thermotherapy has started beingutilized. It is a form of therapy utilizing infrared laser diodefor inducing hyperthermia with a variation between 45°C to60°C. This therapeutic modality is exclusively indicated incases of small melanomas in conjunction with brachytherapyallowing the treatment of lesions > 5 mm, or even as anadjuvant therapy in cases where regression is not achieved orwhere there is a regression of the disease(11).Transpupillary thermotherapy is characterized by a deeppenetration with an immediate cytotoxic effect, resulting innecrosis of the tumor up to 6 mm in depth in experiments withanimals, and 4.7 mm in melanomas of the choroid inhumans(12). The ideal therapy for ocular melanomas still remainscontroversial, and ocular brachytherapy and external radiotherapywith proton beam are adequate therapeutic options aiming atpreserving the ocular globe and thevision(13,14). So, the present study is aimed at analyzing the preliminaryresults from ruthenium-106 brachytherapy in patients with uvealmelanoma.
MATERIALS AND METHODS This is a retrospective study performed on 20 cases ofpatients with unilateral uveal melanoma, referred to theRadiotherapy Unit by the Department of Ophthalmology ofUniversidade Federal de São Paulo, for being submitted toocular brachytherapy, in the period between April 2002 and July2003. The diagnosis was made by clinical and ophthalmologicalexaminations. Fluorescein angiography and ocular ultrasound A-and B-scans were requested. Both modes were employed forevaluating the tumors elevation. For evaluation of the tumorslocalized in the iris and ciliary body ultrasonic biomicroscopywas performed. The staging of patients aiming at characterizing the absenceof metastatic lesions included chest x-ray, abdominal ultrasound,hemogram and biochemical analysis. The patients were stagedaccording to TNM(15) andCOMS(16–18) criteria. For the present study, the following inclusion criteria were taken into consideration: diagnosis of unilateral melanoma of the choroid, iris or ciliary body; tumor < 6 mm in elevation; base diameter < 16 mm; patients with age > 21 years. Patient's informed consent. Exclusion criteria were the following: diagnosis of another malignancy; patients undergoing immunosuppressive therapy; patients incapable of returning for follow-up; patients with severe co-morbidities; multifocal or diffuse lesions with scleral infiltration; previously treated patients; patients with metastasis; lesions that cannot be delimited by ocular ultrasound. Bebig GmbH (Berlin, Germany) ruthenium-106 ophthalmic plaqueswere utilized. The main feature of these plaques isbeta-radiation emission, with maximum 3.54 MeV energy, andhalf-life of 365 days. Two plaque models were employed: COB (round notched) and CCB (round), both with 20 mm in diameter (Figure 2). Patients with lesions proximal to the optic nerve were submitted to brachytherapy with the COB plaque, while in the other the CCB plaque was utilized. Patients with lesions with more than 5 mm in elevation (five patients) were submitted to transpupillary thermotherapy delivered to the apex of the tumor, associated with placement of the ophthalmic plaque. The prescribed radiation dose was calculated on the apex of the lesion, from the inner face of the sclera.
The present study covered the following aspects: demographicdata (age and sex of patients), tumor characteristics (lesionsite, affected eye, TNM and COMS staging, tumor elevation), andtreatment characteristics (type of plaque, radiation dose to theapex, base and sclera). After the treatment, the patients were clinically evaluatedevery three months during the first two years of follow-up, withthe purpose of verifying the local management, progression-freesurvival, the lesion elevation, rate of preservation of theocular globe, visual acuity and presence of metastases andcomplications. Ocular ultrasound and fluorescein angiography wereperformed every three months, and chest x-ray and abdominalultrasound, every six months. The local management was defined asstabilization or decrease in the lesion elevation at imagingstudy in comparison with the pre-treatment study. The presence oflesions in other sites was defined as metastasis.Progression-free survival corresponds to absence of local ordistance failure. Treatment-related adverse effects wereconsidered as complications. The follow-up period started in thedate of the plaque placement. Parameters regarding demographic, tumor and therapycharacteristics, rate of preservation of the ocular globe,presence of metastasis and complications were submitted for adescriptive analysis. With the purpose of comparing numerical variables in relationto the local management, lesion elevation before and after thetherapy, and visual acuity, summary measures were calculated andbox-plots were constructed. The t-test was employed forevaluating variables related to local management and visualacuity, and the paired t-test for comparing pre- andpost-therapy tumor elevation. Contingency tables were elaborated,and the Fisher's exact test was employed for evaluating. Kaplan-Meier curves were constructed to estimate survivalcurves of interest in relation to the progression-freesurvival. In all of the tests, the null hypothesis rejection level wasfixed in 0.05 or 5% (p < 0.05), significant valuesbeing marked with an asterisk, and the non-significant values,with (NS).
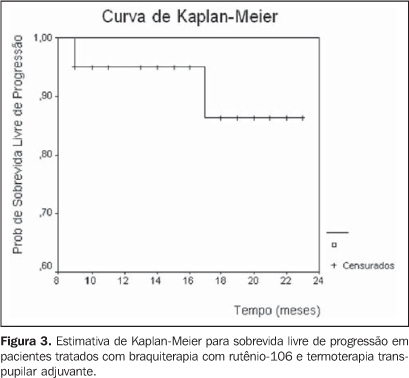
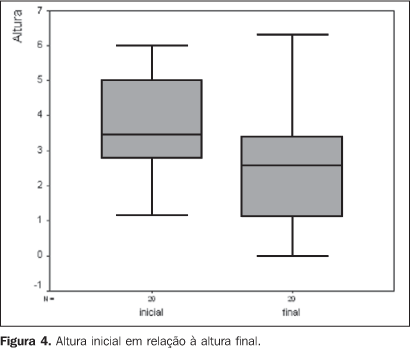
RESULTS The patients' ages ranged between 29 and 80 years (median, 56 years). The majority of patients were Caucasian (95%), and women (70%) (Table 1). As regards tumor characteristics, the right eye was involved in 45%, and the left eye in 55% of cases. Choroid and/or ciliary body melanomas were diagnosed in 85% of patients, and iris melanoma in 15%. The lesion elevation ranged between 1.14 mm and 6 mm (median, 3.45 mm) (Table 1). TMN staging demonstrated 20% of patients staged as T1, and 80% as T2. According to COMS criteria, 10% of lesions were small-sized, and the remaining 90%, intermediate. As regards the therapy, the radiation dose to the apex ranged between 55.8 Gy and 104.8 Gy (median, 84.6 Gy). The median of the radiation-dose to the tumor base and sclera was respectively 248.3 Gy and 319.4 Gy. The CCB plaque was utilized in 75% of cases. The follow-up period ranged between 9 and 23 months (median, 19 months). During the clinical follow-up, an increase in the tumor dimensions was observed in five cases (25%). Of these patients, three were submitted to adjuvant transpupillary thermotherapy, and currently are under follow-up, with no evidence of disease progression. Two patients could not undergo transpupillary thermotherapy because of the lesion localization. One of them is under observation, and enucleation is schedule for the other. Table 2 shows the local management with brachytherapy and associated with transpupillary thermotherapy. The initial elevation of the lesion (p = 0.883), the prescribed radiation-dose to the tumor apex (p = 0.879) and the lesion localization (p = 1.000) did not constitute significant factors for the local management of the disease. The progression-free survival with brachytherapy and with adjuvant transpupillary thermotherapy may be observed in Kaplan-Meier survival curves (Figure 3). One patient progressed to death nine months after therapy due to unrelated causes. Comparing the initial elevation of the lesion with the elevation after the therapy, a significant reduction (p = 0.001) was observed (Figure 4). No patient was submitted to enucleation. As regards visual acuity at the moment of the latest evaluation, 70% of patients presented a visual acuity higher than 20/200, and 30% presented visual acuity equivalent or lower than 20/200. The prescribed radiation dose to the tumor base and sclera did not constitute a prognosis factor of significance for the visual acuity.
During the follow-up period distant metastases were notdetected. Complications were observed in 25% of cases. In fourpatients, visual acuity worsening was observed in one patient,and another presented infectious complication.
DISCUSSION Results regarding demographic compare favorably with previousstudies published in the literature, demonstrating a predominanceof uveal melanoma in Caucasian patients with median age of 60years. Singh and Tophan, in an epidemiological analysis ofmelanomas in the United States reported a 97.8% incidence inCaucasian individuals. In this same study, the ages of thepopulation studied ranged between 6 and 100 years, 59.4 year formen, and 61.5 for women (median, 59.4 years). As regards sex,uveal melanoma seems to present a slightly high incidence in men.Notwithstanding this fact has not been observed in the presentstudy, this may be justified by the low number of patients incomparison with studies performed for incidenceevaluation(1). In the analysis of the tumor characteristics, melanomas mostfrequently were located in the choroid. Also, a higher number ofcases of iris melanomas were observed in the present study inrelation to the literature where the reported incidence achievesonly 2%–3% for lesions localized in thistopography(19). The mild predominance inthe left eye observed in the present study also is reported bysome studies in the literature. Hermann et al. observed that in56% of cases, the tumors were localized in the lefteye(20). However, this difference was notobserved in studies involving a higher number ofcases(18). All of the lesions were staged as T1 or T2N0M0 by the TNM (6thedition)(15), and as intermediate by the COMS.In other publications about the employment of ruthenium-106 inmelanomas therapy, a predominance of tumors staged as T1 and T2also is observed. This is due the fact that ruthenium-106 is aBeta-radiation emitter with low penetrability, besides not beingindicated for more extensive lesions. Also, it may be added thatthe majority of studies was based on the staging methodrecommended by the International Union Against Cancer (UICC) in1997(21), classifying choroid melanomas with> 5 mm in elevation and/or >15 mm in diameter as T3.According to the TMN 6th edition, only those lesions with > 16mm in its larger diameter and/or > 10 mm in elevation would beclassified as T3. So, considering the new staging, one can saythat the incidence of T1 and T2 tumors described in theliterature probably is higher. This also can be confirmed by thecomparison between the median elevation of the lesions in thepresent study (3.45 mm) and that reported in the literature,ranging from 3 mm to 5.2 mm(22,23). The most utilized plaque was the round-shaped CCB. Thegreatest majority of studies performed with ruthenium-106 plaquesdo not describe the plaque models. In the present study, the CCBmodel was utilized in the treatment of patients with centrallesions, proximal to the optic nerve, while the COB plaque wasutilized in the remaining. As regards the lesions localization,the literature reports a lower incidence of central lesions incomparison with paracentral, peripheral or yet ciliary body andiris lesions. In the study performed by the COMS, lesions at 0–2mm from the optic disc corresponded to only 14.9% ofcases(24). On the other hand, in otherpublications, central lesions were observed in 35% of cases,these results being similar to the ones from the presentanalysis(17). The ruthenium-106 plaque time of permanence ranged between twoand five days (median, three days), and was directly proportionalto the prescribed radiation-dose delivered to the tumor apex, andinversely proportional to the plaque activity. The prescribeddose delivered to the tumor apex ranged between 55 Gy and 105 Gy(median, 85 Gy), and to the base, the prescribed dose rangedbetween 148 Gy and 450 Gy. The dose delivered to the scleraranged between 191 Gy and 578 Gy. The radiation dose to beprescribed to the tumor apex with ruthenium-106 is still to bedefined in the literature. In an analysis of several studies inthe literature about the use of ruthenium plaque for treatment ofmelanomas, one can observe that most of times the dose rangesbetween 80 Gy and 100 Gy, but studies with doses from 60 Gy to160 Gy may be found. Rouberol et al. have treated 213 patientsaffected by choroid and ciliary body melanoma with a 60 Gydose(25). Shields et al., in a studyabout visual acuity in 1,106 patients with uveal melanomasubmitted to brachytherapy with iodine-125, ruthenium-106,cobalt-60 or iridium -192, have described a median dose of 90.9Gy to the apex, and 330 Gy to the tumorbase(26). Seregard, in a meta-analysis with1,066 patients treated with ruthenium-106 has observed a dose tothe apex ranging between 80 Gy and 100Gy(27). A study describing the Dutchexperiment with ruthenium for uveal melanoma utilized doses up to160 Gy delivered to the tumor apex, and doses to the sclera from220 Gy to 950 Gy(3). Hermann et al., analyzingthe effect of the dose-assignment in relation to results fromruthenium-106 plaque brachytherapy, utilized doses of 120 Gy tothe apex of the tumor(20). Shields et al., in astudy with concomitant transpupillary thermotherapy andbrachytherapy, describe a dose to the apex ranging between 55 Gyand 124 Gy (median, 90 Gy), and 254 Gy to thebase(11). As regards radiation dose to beutilized, the American Brachytherapy Society recommends the doseof 85 Gy to the apex of the lesion when iodine-125 is utilized asradioisotope(14). However, these sameguidelines recommend a dose from 120 Gy to 160 Gy aiming atmaximizing the healing effect when rutheium-106 is utilized.Meanwhile, the American Brachytherapy Society suggests that thislarge dose gradient may result in high doses to the sclera, whichmay cause a higher number ofcomplications(14). In a randomizedprospective study developed by the COMS utilizing iodine-125 inthe treatment of choroid melanomas, in 49.9% of cases, the doseto the apex was 85.1 Gy to 120 Gy, and in 41.3% of patients thedose to the sclera ranged between 293 Gy and 409.9Gy(27). So, studies evaluatingdose-assignment excepted, the doses utilized in the present studyare compatible with data in the literature regarding prescribedradiation dose to the apex of the lesion, to the tumor base andsclera. Our results in relation to local management andprogression-free survival compare to previous studies, despitethe difficulty to evaluate retrospective data from differentcenters, considering variations in follow-up periods, tumorsdimensions and doses delivered. Summanen et al., evaluating 100patients treated with ruthenium-106, have observed local failurein 19% of cases with 0.1 to 2.7 years of follow-up (median, 0.7year)(28). This leads to the conclusion thatlocal failures occur precociously, especially in the first yearsof follow-up, and are very significant for the interpretation ofour results. Studies with similar follow-up periods demonstrate acomplete tumor regression in 57.1% of cases and localrecidivation in 31% of patients(29).Foerster et al., evaluating the ruthenium therapy in100 patientsaffected by uveal melanoma, have observed local recidivation of12%, with a median two-year follow-upperiod(30). Seregard, in a review of theSwedish experiment with transpupillary thermotherapy as anadjunct to ruthenium brachytherapy, has observed in a 20-monthfollow-up period that in 70.3% of cases there was a partial orcomplete regression of the lesion, a result very similar to the75% local management observed in the presentstudy(31). Lommatzsch et al., analyzing 141 patients submitted toruthenium brachytherapy at a dose of 100 Gy, have observed adisease-free survival rate corresponding to 89.6% in fiveyears(32). Seregard, in a meta-analysisinvolving 1,066 patients treated with ruthenium at doses between80 Gy and 100 Gy, has observed a disease-free survival of 86% infive years(27). In the present study, once theprogression-free survival of patients submitted to brachytherapyand transpupillary thermotherapy is analyzed, one may observethat our results are similar to those in the literature, with alonger follow-up period and higher number of cases. Studies evaluating the dose-assignment effect on the localmanagement show controversial results. Tjho-Heslinga et al.,utilizing a dose to apex of 160 Gy in 101 patients affected byuveal melanoma, have observed complete and partial remission inrespectively 62.6% and 31.3% of cases, these results beingslightly superior to those described in the literature with theusual doses. In the present study, transpupillary thermotherapyalso was utilized in 25 patients, allowing a reduction in theradiation dose and, consequently, in the risk ofretinopathy(3). However, in anotherstudy with a 120 Gy dose to the apex of the lesion in patientswith choroid melanomas, the benefit from the dose-assignment tothe survival has not been observed(20). In a literature review, elevation and larger basal diameter ofthe tumor, dose delivered to the tumor apex and retinaldetachment play a significant role in relation to the localmanagement and disease-freesurvival(27). In the present study,factors like lesion elevation, radiation dose, and tumorlocalization did not affect the local management of the disease.This may have occurred because of the number o patients andnumber of failures observed, since this is a rare malignantneoplasm. Also, it may be added that the concomitant employmentof transpupillary thermotherapy and brachytherapy in patientswith lesions > 5 mm may have contributed to the favorable andsimilar results of patients with larger lesions who receivedlower radiation dose to the apex of their tumors. The post-therapy tumor elevation was significantly lower than that before the treatment, evidencing that the therapy was effective, allowing a decrease in the tumor thickness of about 65% of the initial elevation. In a literature review in terms of reduction of tumor elevation after ruthenium brachytherapy, a reduction around 3%/month is observed, and the majority of lesions do not present a complete regression, stabilizing at a constant value around 61% of the initial elevation after approximately 24 months(23). In our series, results concerning enucleation and visual acuity are slightly better than those in the literature. . Quivey et al. have observed that 58% of 239 patients treated with iodine plaques maintained a vision > 20/200(33). The COMS Report no. 16 has evaluated the visual acuity of patients three years after iodine brachytherapy and observed an enucleation rate of 6.2% and visual acuity < 20/200 in 43% of cases. In this same study, after a 24-month follow-up, compared with our series, 4% of the patients had been enucleated, and 33% presented visual acuity < 20/200(24). In another analysis of visual acuity after brachytherapy, Shields et al. have observed that in 51% of cases the visual acuity was < 20/200(26). Notwithstanding our results have shown that the prescribed radiation dose does not affect the visual acuity, probably because of the number of cases, the factors that seem to be related to the visual acuity after the therapy are: the isotope utilized, the presence of diabetes, the tumor elevation, the lesion localization, the type of plaque utilized, and the presence of retinal detachment(24). The presence of metastasis is observed in about 10%-20% of patients with uveal melanoma, and is related mainly to the tumor size(34). In our series, the presence of metastasis was not observed. This may be explained by the fact that the present study includes only patients at the initial phase of the disease, with tumors < 6 mm in elevation. Analyzing this series as a whole, despite the short follow-upperiod and the small number of patients, our results show thatruthenium brachytherapy allowed a good local management of thedisease and a reasonable progression-free survival in the studiedpopulation. Notwithstanding enucleation would be the therapyindicated in our institution, the use of this radioisotopeallowed, in all of the cases, the preservation of the ocularglobe, and the maintenance of the visual acuity at a certainextent. This is the first study about the use of ruthenium in ourcountry, and we consider that this radioisotope may beexclusively utilized in the treatment of uveal melanomas with upto 5 mm in elevation, while patients with lesion with more than 6mm in elevation are candidates to brachytherapy with otherradioisotopes. In cases of melanomas with 5 mm to 6 mm inelevation, ruthenium brachytherapy must, if possible, beassociated with transpupillary thermotherapy.
REFERENCES 1. Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology 2003;110:956–961. [ ] 2. Shields JA, Shields CL. Introduction to melanocytic tumors of the uvea. In: Shields JA, Shields CL, editors. Intraocular tumors: a text and atlas. Philadelphia: Saunders, 1992;45–60. [ ] 3. Tjho-Heslinga RE, Davellar J, Kemme HM, et al. Results of ruthenium irradiation of uveal melanomas: the Dutch experience. Radiother Oncol 1999;53:133–137. [ ] 4. Moore RF. Choroidal sarcoma treated by the intraocular insertion of radon seeds. Br J Ophthalmol 1930;14:145–146. [ ] 5. Stallard HB. Radiotherapy for malignant melanoma of the choroid. Br J Ophthalmol 1966;50: 147–155. [ ] 6. Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No.18. Arch Ophthalmol 2001;119:969–982. [ ] 7. Lommatzsch PK. Results after b-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years' experience. Br J Ophthalmol 1986;70:844–851. [ ] 8. Lommatzsch PK, Werschnik C, Schuster E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefes Arch Clin Exp Ophthalmol 2000;238:129–137. [ ] 9. Bebig Isotopen-und Medizintechnick GmbH. Aplicadores oftalmológicos rutênio-106: instruções de emprego. Berlim: Bebig, 2001;04:01–08. [ ] 10. Summanen P, Immonen I, Kivela T, Tommila P, Heikkonen J, Tarkkanen A. Radiation related complications after ruthenium plaque radiotherapy of uveal melanoma. Br J Ophthalmol 1996;80:732–739. [ ] 11. Shields CL, Cater J, Shields JA, et al. Combined plaque radiotherapy and transpupillary thermotherapy for choroidal melanoma: tumor control and treatment complications in 270 consecutive patients. Arch Ophthalmol 2002;120:933–940. [ ] 12. Journée-de Korver JG, Oosterhuis JA, de Wolff-Rouendaal D, Kemme H. Histopathological findings in human choroidal melanomas after transpupillary thermotherapy. Br J Ophthalmol 1997; 81:234–239. [ ] 13. Gragoudas ES, Goitein M, Verhey L, Munzenreider J, Suit HD, Koehler A. Proton beam irradiation. An alternative to enucleation for intraocular melanomas. Ophthalmology 1980;87:571–581. [ ] 14. Nag S, Quivey JM, Earle JD, et al. The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys 2003;56:544–555. [ ] 15. Greene FL, Page DL, Fleming ID, editors. In: AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag, 2002;377–382. [ ] 16. The Collaborative Ocular Melanoma Study Group. Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. Arch Ophthalmol 1997;115:1537–1544. [ ] 17. Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, II: characteristics of patients enrolled and not enrolled. COMS Report No. 17. Arch Ophthalmol 2001;119:951–965. [ ] 18. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma I: characteristics of patients enrolled and not enrolled. COMS Report No. 9. Am J Ophthalmol 1998;125:767–778. [ ] 19. Shields CL, Shields JA, Materim M, Gershenbaum E, Singh AD, Smith A. Iris melanoma: risk factors for metastasis in 169 consecutive patients. Ophthalmology 2001;108:172–178. [ ] 20. Hermann RM, Pradier O, Lauritzen K, Ott M, Schmidberger H, Hess CF. Does escalation of the apical dose change treatment outcome in b-radiation of posterior choroidal melanomas with 106Ru plaques? Int J Radiat Oncol Biol Phys 2002;52: 1360–1366. [ ] 21. Sobin LH, Wittkind C. International Union Against Cancer. TNM classification of malignant tumors. 5th ed. New York: Wiley-Liss, 1997. [ ] 22. Stoffelns BM, Kutzner J, Jochem T. Retrospective analysis of ruthenium-106 brachytherapy for small and medium-sized malignant melanoma of the posterior choroid. Klin Monatsbl Augenheilkd 2002;219:216–220. [ ] 23. Kaiserman I, Anteby Y, Chowers I, Blumenthal EZ, Kliers E, Pe'er J. Changes in ultrasound findings in posterior uveal melanoma after ruthenium 106 brachytherapy. Ophthalmology 2002;109: 1137–1141. [ ] 24. Melia BM, Abramson DH, Albert DM, et al. Collaborative Ocular Melanoma Study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years. COMS Report No. 16. Ophthalmology 2001;108: 348–366. [ ] 25. Rouberol F, Roy P, Kodjikian L, Gerard JP, Jean-Louis B, Grange JD. Survival, anatomic, and functional long-term results in choroidal and ciliary body melanoma after ruthenium brachytherapy (15 years' experience with beta-rays). Am J Ophthalmol 2004;137:893–900. [ ] 26. Shields CL, Shields JA, Cater J, et al. Plaque radiotherapy for uveal melanoma: long-term visual outcome in 1106 consecutive patients. Arch Ophthalmol 2000;118:1219–1228. [ ] 27. Seregard S. Long-term survival after ruthenium plaque radiotherapy for uveal melanoma. A meta-analysis of studies including 1,066 patients. Acta Ophthalmol Scand 1999;77:414–417. [ ] 28. Summanen P, Immonen I, Heikkonen J, Tommila P, Laatikainen L, Tarkkanen A. Survival of patients and metastatic and local recurrent tumor growth in malignant melanoma of the uvea after ruthenium plaque radiotherapy. Ophthalmic Surg 1993;24:82–90. [ ] 29. Busse H, Muller RP. Techniques and results of 106Ru/106Rh radiation of choroidal tumours. Trans Ophthalmol Soc UK 1983;103(Pt 1):72–77. [ ] 30. Foerster MH, Bornfeld N, Wessing A, Schulz U, Schmitt G, Meyer-Schwickerath G. Treatment of malignant melanomas of the uvea with 106-ruthenium applicators. Report on the first 100 Essen cases. Klin Monatsbl Augenheilkd 1984;185: 490–494. [ ] 31. Seregard S, Landau I. Transpupillary thermotherapy as an adjunct to ruthenium plaque radiotherapy for choroidal melanoma. Acta Ophthalmol Scand 2001;79:19–22. [ ] 32. Lommatzsch PK, Werschnik C, Schuster E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefes Arch Clin Exp Ophthalmol 2000;238:129–137. [ ] 33. Quivey JM, Char DH, Phillips TL, Weaver VA, Castro JR, Kroll SM. High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol Biol Phys 1993;26:613–618. [ ] 34. Kleineidam M, Guthoff R, Bentzen SM. Rates of local control, metastasis, and overall survival in patients with posterior uveal melanomas treated with ruthenium-106 plaques. Radiother Oncol 1993;28:148–156. [ ]
Received February 22, 2006. Accepted after revision August 21, 2006.
* Study developed in the Radiotherapy Unit of Department of Medicine and Department of Ophthalmology at Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554