Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 2 - Mar. / Apr. of 2007
Vol. 40 nº 2 - Mar. / Apr. of 2007
|
WHICH IS YOUR DIAGNOSIS?
|
|
Which is your diagnosis? |
|
|
Autho(rs): Marcelo Souto Nacif, Amarino Carvalho de Oliveira Júnior, Denise Madeira Moreira, Mônica Regina Nagano, José Hugo Mendes Luz, Marcio dos Santos Martins, Mauro Esteves de Oliveira, Luiz Fernando Mendes, Carlos Eduardo Rochitte |
|
|
IProfessor at Faculdade de Medicina de Teresópolis (Unifeso), Teresópolis, RJ, Brazil, Sub-coordinator for Post-graduation at Instituto de Pós-Graduação Médica Carlos Chagas (IPGMCC), Doctorate Student in Radiology (Cardiac Magnetic Resonance Imaging) at Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil
A female, 67-year old patient weighting 80 kg, with 1.53 m inheight, coming from Minas Gerais state, has been referred to theService of Radiology and Diagnostic Imaging at HospitalPró-Cardíaco to be submitted to magnetic resonanceimaging (MRI) of the heart. Description of images Figure 1. Images acquisition with ECG-gating, in cine-Fiesta sequence (SSFP), at end-diastole, in the following planes: four-chamber (A), middle, basal short axis (B), map of segmental function (C) and aortic flow curve (D). Observe normal-sized atria, normal right-ventricular volume with preserved ventricular function. There is a mild dilatation of the left ventricle, with thinning of the medial and basal, infero-lateral walls, a slight global left-ventricle (LV) dysfunction, estimated ejection fraction of 47.1%. Also, akinesia was identified in the medial, infero-lateral segment, hypokinesia in the basal, infero-lateral and medial antero-lateral segments, with normokinesia in the other segments. Note the typical aspect of apical aneurysm and mild aortic insufficiency. Figure 2. Images acquired with ECG-gating. Delayed enhancement, middle basal short-axis (A), two long axis two-chamber perpendicular to each other (B), and map of segmental delayed enhancement (C). Observe that there was a delayed myocardial enhancement (< 50% of segmental area) in the medial antero-lateral and basal infero-lateral segments, and delayed intramural enhancement (> 75% of the segmental area) in the medial, infero-lateral segment. Diagnostic: Chagasic cardiomyopathy.
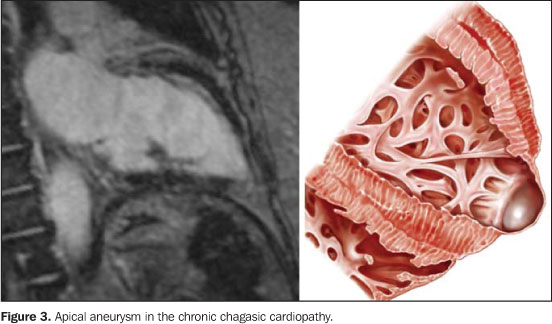
COMMENTS Firstly described by Carlos Chagas in 1909(1),American trypanosomiasis or Chagas disease — a conditioncaused by the Trypanosoma cruzi, a protozoan parasite of theMastigophora class, order Kinetoplatida and Trypanosomatidaefamily — is a highly prevalent antropozoonosis, with highmorbidity in the Latin America, and is considered as one of theparasitic infectious diseases with higher incidence in thecontinent(2,3). As a matter of fact, overallprevalence of Chagas disease in Latin American countries isestimated at 16 to 18 million cases, and about 100 million peopleat risk of contracting the infection(4,5). The pathological findings are highly suggestive of thedisease. Macroscopically, chronic chagasic cardiopathy ischaracterized by a progressive, chronic myocarditis with increasein the myocardial muscle— which may reach more than 1,000grams —, leading to the four cavities dilatation, and givingthe heart a globoid appearance(6,7). Usually, themyocardium presents softened, with irregular thickness and areaswhere the thickness of the wall is decreased, and others were thewall is hypertrophic. The pericardium may present smooth, bright,transparent, or thickened. Frequently, the valvular endocardiumis not involved, except when there is a remarkable dilatation ofthe ring with dysfunction and insufficiency, originating valvularfibrous thickening. Microscopically, the presence of chronicmyocarditis in association with fibrosis is observed, in somecases with a granulomatous component (separation of the fiberswith formation of pseudofollicular structures with epithelioidcells and giant, multinuclear, non-parasited cells), amastigotenests being rare. Cardiac cells demonstrate several alterations,such as vacuolation, accumulation of lipofuscin granules, hyalinedegeneration, intracellular edema and myofibrilardisorganization(6,7). Chronic chagasic cardiopathy manifestations will depend on thedegree of myocardial function involvement. So,clinical-pathological presentations are the following: 1) Cardiac failure syndrome (CFS). One of the lesions typically related to CFS — but also to thromboembolic alterations — is the apical aneurysm that may range from a fibrous thinning to true aneurysmal formations resulting from sacular dilatation with few millimeters in diameter (up to 5 cm), whose wall is some times constituted uniquely by the union between endocardium and pericardium (Figure 3).
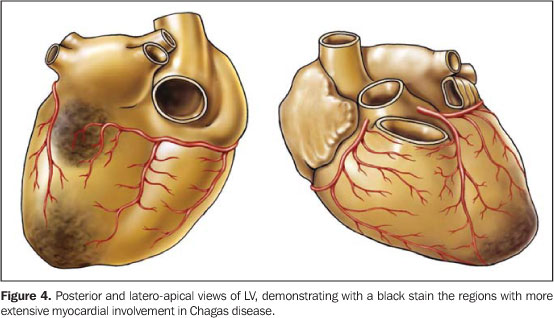
2) Arrhythmic syndrome whose pathological substrate includesinvolvement of the sinoatrial node, atrioventricular node andbundle of His, with the following main alterations: a) disordersof atrio- and intraventricular conduction; b) functional disorderof the sinusal node; c) primary and secondary ventricularrepolarization abnormalities; d) fibrosis and inflammation; e)autonomic dysfunction; f) endothelial and coronary dysfunction.All of these disorders may lead to difficult-to-managearrhythmias(8). Sudden death predominates as frequentcardiac cause of death in all presentations of the disease inendemic areas, and is erroneously diagnosed as acute coronarysyndrome. 3) Thromboembolic syndrome, a frequent finding in patientswith CFS, although rarely diagnosed, is a factor responsible forboth the refractoriness to treatment and a higher risk for death.This syndrome probably is a result of: a) stasis secondary tocardiac dilatation; 2) arrhythmias; 3) endocardial fibrosis; 4)mural endocarditis. Most affected systemic organs in decreasingfrequency order are the kidneys, the spleen and thebrain(9,10). Cardiac magnetic resonance imaging contribution in Chagasdisease Cardiac involvement is a determining factor in the Chagasdisease prognosis. Therefore, an appropriate evaluation of theheart is essential in the management of the patient.Approximately 30% to 40% of infected peoples will develop cardiacabnormalities during their lives, but only 10% to 20% willpresent the symptomatic form of the disease. Histopathological studies indicate the presence of mild chronic myocarditis manifested by the scattered mononuclear cell infiltrate with the surrounding myocytes undergoing several degrees of degeneration and necrosis. The focal and diffuse fibrosis is prominent in the myocardium and conduction system. Myocardial fibrosis has been detected in several anatomopathological studies for Chagas disease where the prevalent sites for fibrosis and left ventricular (LV) microcirculation abnormalities were the LV apex and the basal, infero-lateral segment (Figure 4).
Similarly to data found in the literature,the segmentalanalysis by MRI and the delayed myocardial enhancement clearlydemonstrate in vivo that the LV apical and infero-lateralregions are the most frequent sites for myocardial fibrosis inpatients with Chagas disease(11,12). Thiscorroborates the concept that in the Chagas disease, myocardialfibrosis is frequently related to regions of terminal circulationlike the apex (terminal circulation between the anteriordescendent coronary artery and descendent posterior) and thebasal, infero-lateral segment (terminal circulation between theright coronary artery and the left circumflexartery)(11,12). Future prospects Recent technological developments in cardiac magneticresonance imaging have been utilized to detect and quantify earlysigns of cardiac involvement in Chagas disease. These signs mayconstitute significant prognostic information. This has alreadybeen routinely made by means of a detailed evaluation of thecardiac function with the myocardial tagging technique,High-resolution cine-MRI, and delayed enhancement for detectionof myocardial fibrosis. Cardiac MRI study in Chagas disease willinclude acquisitions in a single breath-hold with cine-3D, thetechniques of myocardial perfusion, edema and myocardialinflammation detection, and monitoring of intramyocardialstem-cell injections for treatment of the chagasiccardiomyopathy. Our view is that cardiac MRI may be utilized in the future forselection of patients with very early myocardial involvement bythe Chagas disease. With this data, we can develop newtherapeutic methods to change the natural history of the Chagasdisease.
REFERENCES 1. Maguire JH. Chagas' disease – can we stop the deaths? N Engl J Med 2006;355:760–761. 2. Siqueira-Batista R, Moraes HP, Hahn MD. Patogenia e patologia. In: Siqueira-Batista R, Corrêa AD, Gomes AP, Gerull B, editores. Moléstia de Chagas. Rio de Janeiro: Rubio, 2007. 3. Rassi A Jr, Rassi A, Little WC, et al. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med 2006;355:799–808. 4. Rochitte CE, Tassi EM, Shiozaki AA. The emerging role of MRI in the diagnosis and management of cardiomyopathies. Curr Cardiol Rep 2006;8:44–52. 5. Rochitte CE, Oliveira PF, Andrade JM, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with Chagas' disease: a marker of disease severity. J Am Coll Cardiol 2005;46:1553–1558. 6. Edelman RR, Hesselink JR, Zlatkin MB, Crues JV III. Clinical magnetic resonance imaging. 3rd ed. Philadelphia: Saunders-Elsevier, 2006. 7. Higuchi ML, Benvenuti LA, Martins RM, Metzger M. Pathophysiology of the heart in Chagas' disease: current status and new developments. Cardiovasc Res 2003;60:96–107. 8. Higuchi ML, Fukasawa S, De Brito T, Parzianello LC, Bellotti G, Ramires JA. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas' disease: a three dimensional confocal microscopy study. Heart 1999;82:279–285. 9. Kalil R, Bocchi EA, Ferreira BM, et al. Magnetic resonance imaging in chronic Chagas cardiopathy. Correlation with endomyocardial biopsy findings. Arq Bras Cardiol 1995;65:413–416. 10. Pereira Barreto AC, Arteaga E, Mady C, Ianni BM, Bellotti G, Pileggi F. Sexo masculino. Fator prognóstico na doença de Chagas. Arq Bras Cardiol 1993;60:225–227. 11. Kim RJ, Judd RM. Gadolinium-enhanced magnetic resonance imaging in hypertrophic cardiomyopathy: in vivo imaging of the pathologic substrate for premature cardiac death? J Am Coll Cardiol 2003;41:1568–1572. 12. Macedo R, Schmidt A, Rochitte CE, Lima JA, Bluemke DA. MRI to assess arrhythmia and cardiomyopathies. J Magn Reson Imaging 2006;24: 1197–1206.
Study developed at Hospital Pró-Cardíaco, Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554